At the University of Tokyo (UTokyo), scientists have identified a new group of proteins that have the shape and potential to defend against protein clumps linked with neurodegenerative diseases in laboratory experiments.
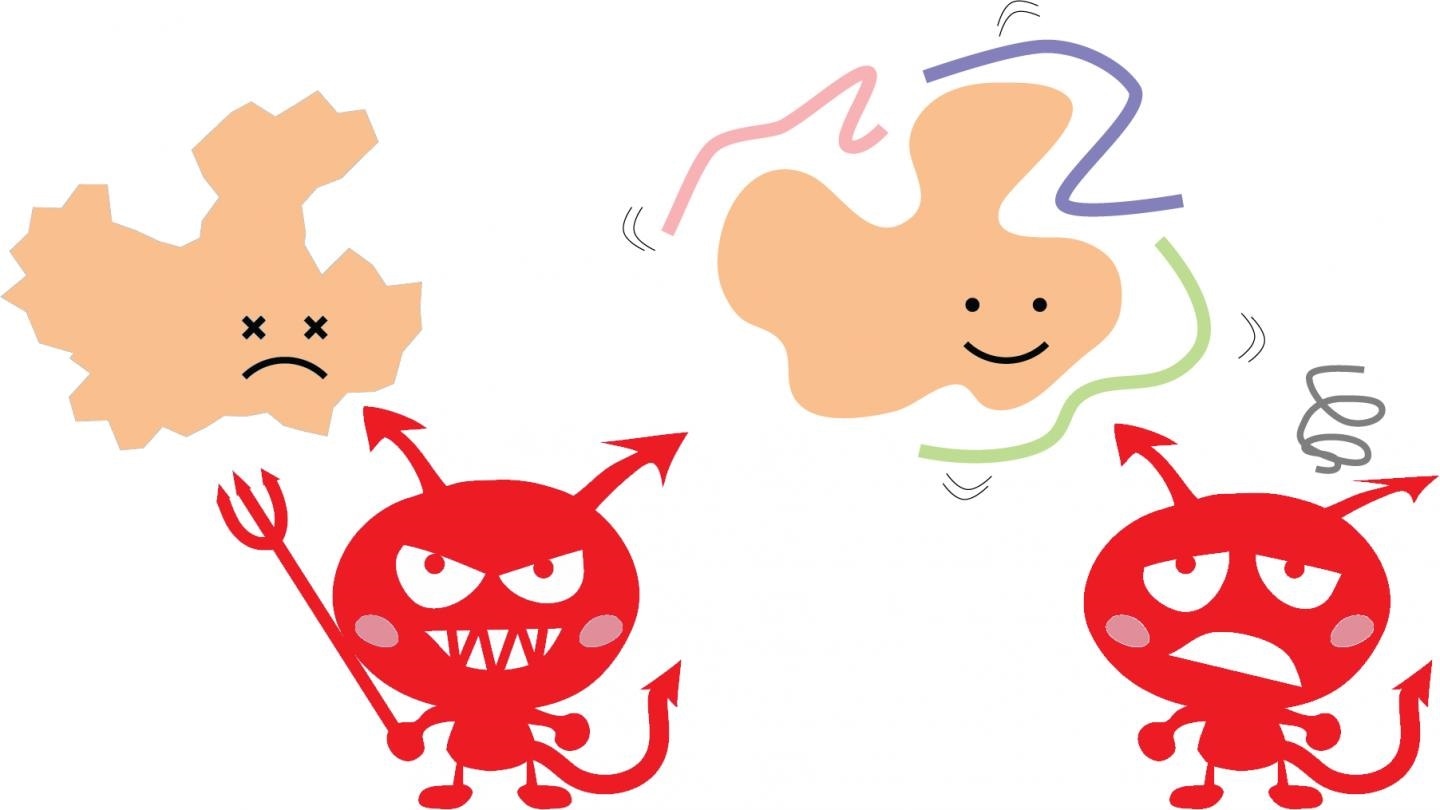
Damage (red devils) like drying out, harsh chemicals or heat normally causes proteins to become unstable and lose their proper shape and function (left side, orange). Researchers at the University of Tokyo have characterized Hero proteins (pink, purple, green), long, flexible proteins that protect other proteins (right side, orange). Image Credit: Kotaro Tsuboyama, CC BY 4.0.
The Hero proteins are resistant to heat and are prevalent in insects, animals, and human beings.
While a majority of the proteins are known to have well-defined twists and folds that create a stiff structure, the new type of protein has a flexible and long string-like structure.
Scientists had initially discovered the first of these unusual proteins in flies and dubbed them by utilizing a combination of an informal Japanese term implying not rigid or weak and the small suffix usually connected to young boys’ names like “hero-hero kun.” Scientists realized years later that this name also symbolizes the English meaning “hero”—a brave defender.
Now, the UTokyo researchers have reported that Hero proteins can protect other types of proteins, prolong the life span of fruit flies by 30%, and protect fruit flies as well as laboratory-grown human motor neurons from harmful protein clumps, such as those that are found in patients with neurodegenerative diseases.
An unlikely discovery
The Hero proteins were accidentally discovered in roughly 2011 when Shintaro Iwasaki, who was a graduate student at that time, came across a strange heat-resistant protein that boosted the stability of Argonaute—a protein that is prominent in the laboratory’s studies. Iwasaki currently heads his own laboratory at RIKEN.
It was kind of cool to know that a strange, extremely disordered, heat-resistant protein improved the behavior of Argonaute, but its biological relevance was unclear and, moreover, the protein’s sequence seemed unrelated to anything else. So, we didn’t know what to do next and just decided to put it on the shelf until years later.”
Yukihide Tomari, Professor and Research Laboratory Leader, University of Tokyo
Tomari is also the last author of the study published in the PLOS Biology journal.
Ultimately, Kotaro Tsuboyama observed the hero-hero Kun protein in a new light, after joining the laboratory as a doctoral student and currently as a postdoctoral researcher.
Heroes in disguise
Proteins that have analogous functions often have similar amino acid sequences even between diverse species, which is referred to as evolutionary conservation by experts.
When initially discovering the hero-hero Kun proteins, Tomari’s research team found the lack of evolutionary conservation in the proteins. This appears to be a defining trait for Hero proteins, rendering it hard to predict not only their function but also their identity.
To reveal the real identities of additional Hero proteins, scientists cultured the cells of fruit flies and humans in the laboratory, produced extracts from the cells, and then merely boiled them.
Chemical interactions that support the structure of a protein are usually weakened by increased temperatures, and this causes the proteins to unfold and bind together with other unfolded proteins.
Proteins are generally damaged by heat, but we found that Hero proteins remain intact even at 95 degrees Celsius [203 degrees Fahrenheit] without losing function. It is a bit strange, which is why I think no one has carefully characterized these proteins before.”
Kotaro Tsuboyama, Postdoctoral Researcher, University of Tokyo
The scientists then utilized an analytical method known as mass spectrometry to detect if any protein was left in the boiled test tubes, and observed hundreds of Hero proteins in humans and fruit flies.
Heroes to the rescue
Tsuboyama chose six Hero proteins to analyze comprehensively. He combined a few of the six Hero proteins with other “client” proteins. Those clients retained their function and shape in spite of dryness, extreme heat, or aggressive chemicals that would usually kill them.
In experiments involving laboratory-grown human motor nerve cells, high concentrations of Hero proteins prevented the cells from forming the protein clumps—a trait of amyotrophic lateral sclerosis (ALS), which is a neurodegenerative disease—and restored their normal patterns of growth.
The huge and sensitive eyes of fruit flies are generally regarded as disease models, as these eyes are deformed by mutations that are responsible for causing neurodegeneration in humans.
The scientists found that when the activity of Hero proteins was enhanced, it protected the eyes of the flies from deformation. This deformation was induced by protein clumps linked with ALS. On the other hand, the removal of normal Hero activity promoted defects during the development of the fly eye.
In addition, the scientists found a proof that when healthy fruit flies were genetically modified to have high concentrations of individual Hero proteins all through their whole bodies, these proteins were able to support the flies’ longevity. Interestingly, a few Hero proteins extended the life span of flies by approximately 30%.
“It appears that Hero proteins naturally exist to keep other proteins happy,” added Tomari.
To be continued ...
We saw many positive effects, but so far, we did not find any ‘superhero’ among those six Hero proteins that can stabilize all client proteins. Some Hero proteins are good for some clients, and others are good for other clients.”
Kotaro Tsuboyama, Postdoctoral Researcher, University of Tokyo
The team is now planning further experiments to detect any rules or patterns about which Hero proteins can aid the client molecules present in living organisms.
“We hope that, in the long run, Hero proteins can be useful for biotechnological and therapeutic applications,” concluded Tomari.
Source:
Journal reference:
Tsuboyama, K., et al. (2020) A widespread family of heat-resistant obscure (Hero) proteins protect against protein instability and aggregation. PLOS Biology. doi.org/10.1371/journal.pbio.3000632.