DNA is a genetic material that continues to survive only through replication, either naturally or synthetically.
Humans require reproduction of DNA to replace damaged or old cells, but the potential to replicate DNA in a laboratory setting allows scientists to understand the disease mechanisms or the platform to develop treatments.
A newly developed low-cost method provides a highly efficient way to reduce DNA errors. Image Credit: ZHANG Jia.
Furthermore, large-scale production of DNA through such replication is the foundation of synthetic biology. But quite similar to the way people write or type an article, the process of DNA “writing” is prone to errors. This has turned out to be a major concern in the large-scale synthesis of DNA.
At present, a research team from the Chinese Academy of Sciences (CAS) has created a more cost-effective and efficient technique to accurately replicate DNA compared to conventionally used techniques. The study outcomes were reported in ACS Synthetic Biology on March 5th, 2020.
In synthetic biology, genes, gene networks and even entire genomes are synthesized to create new functions.”
ZHANG Jia, Study First Author and Researcher, Single-Cell Center, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
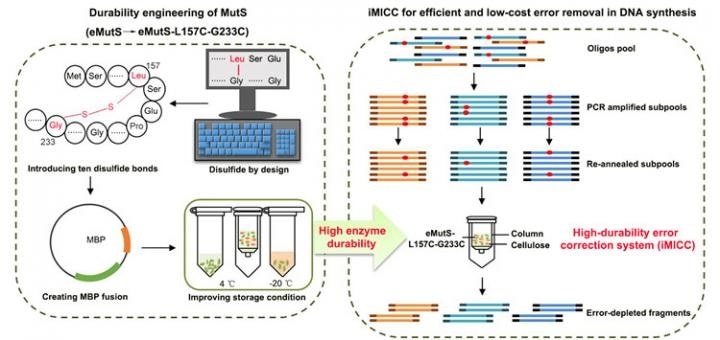
In the DNA replication process, DNA is assembled in a liquid or on a microchip, provided for the deliberate pairing of particular genetic fragments. According to ZHANG, the difficulty is that the fragments often mismatch, generating considerable errors.
Existing techniques to minimize these errors involve the use of a protein named MutS, which tends to get attracted to mismatched genetic fragments. The protein serves as a flag on the errors, enabling researchers to find and eliminate them.
However, this process is expensive and time-intensive. This is mainly because the key error-correcting enzyme of eMutS, which is a protein derived from E. coli that binds errors with high precision, is fragile and cannot endure this process for a long time.
To tackle this challenge, we have developed a simple, effective and cost-efficient error-correction system that is readily applicable in gene synthesis workflow.”
ZHANG Jia, Study First Author and Researcher, Single-Cell Center, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
The CAS researchers started by using chemical stabilizers to treat eMutS, where the stabilizers were in the form of a type of molecular glue named disulfide bonds. The introduced bonds had a strong chemical structure, and with enhancements to enzyme production and storage, they extended the shelf-life of the proteins from 7 to 63 days.
It is quite time-consuming to prepare the proteins for the error-removal process, so this exceptional increase in the durability of the enzyme shows that researchers can switch from protein preparation once a week to once in two months. In terms of industry-scale DNA synthesis workflows, this translates to a considerable reduction in labor, operation, and time costs.
Moreover, when the new and more durable eMutS protein is used, 86.4% of the synthetic DNA fragments are error-free—this is an almost seven-fold increase in accuracy from the commercial enzyme systems available on the market at present, says ZHANG.
This system’s high fidelity, simple operation and low cost in error correction address one of the key challenges in DNA synthesis and could have implications for broad applications in synthetic biology, including industrial applications.”
XU Jian, Study Senior Author and Director, Single-Cell Center, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
The researchers intend to continue optimizing the life of the proteins, while enhancing the accuracy of eliminating the error in DNA synthesis.
Source:
Journal reference:
Zhang, J., et al. (2020) Efficient and Low-Cost Error Removal in DNA Synthesis by a High-Durability MutS. ACS Synthetic Biology. doi.org/10.1021/acssynbio.0c00079.