Novel biomaterials could soon be created using magnetic bacteria.
Headed by Professor Dr. Dirk Schüler, a group of microbiologists at the University of Bayreuth has created an innovative, modular system to genetically reprogram bacteria and thus change these microorganisms into cell factories for multifunctional magnetic nanoparticles that integrate numerous useful properties and functions.
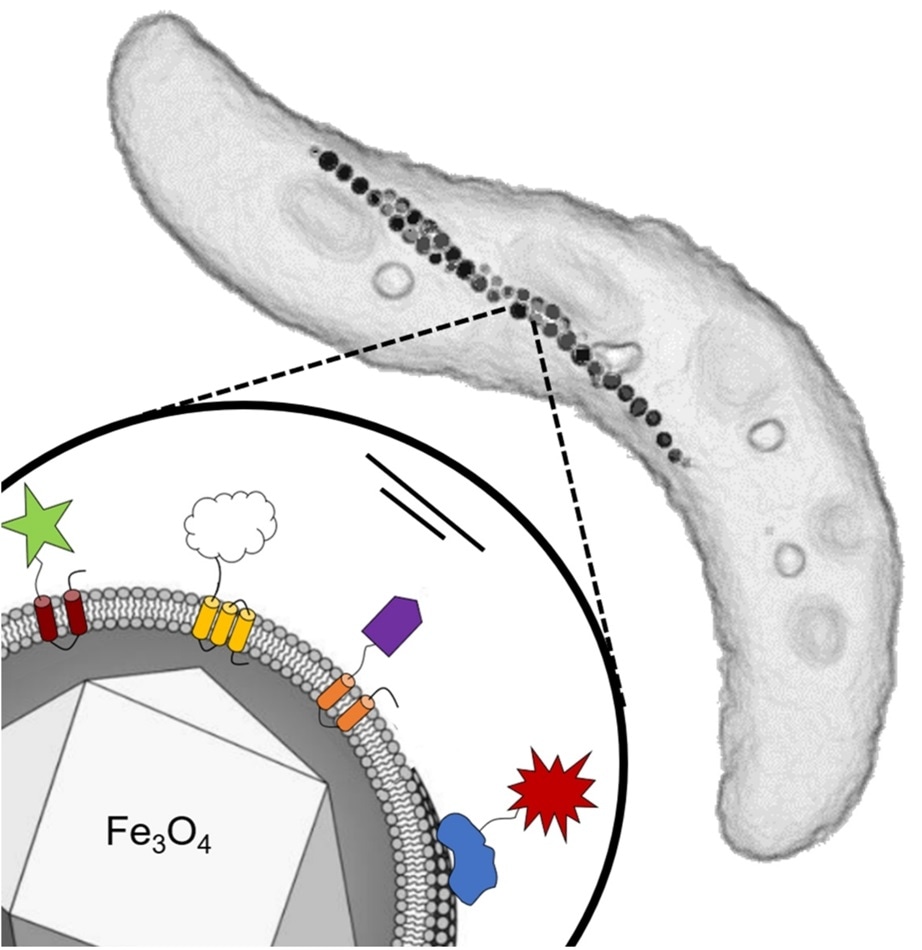
Top right: Schematic of a cell of the magnetic bacterium Magnetospirillum gryphiswaldense used in this study, showing its intracellular chain of magnetosome particles. Each cell has a length between three and five micrometers. Bottom left: Single magnetosome with an iron oxide core enveloped by a membrane. Different functional groups from foreign organisms were genetically fused to specific proteins of the magnetosome membrane. Image Credit: Frank Mickoleit/Clarissa Lanzloth.
Magnetic nanoparticles exhibit good biocompatibility and excellent magnetic properties, making them a potential, new material in the biotechnological and biomedical field. The research team has described the findings in the Small journal.
From magnetosomes to versatile nanoparticles
Magnetic bacteria that belong to Magnetospirillum gryphiswaldense species align their swimming behavior along the magnetic field of Earth. Inside the cells, magnetic nanoparticles—referred to as magnetosomes—are organized in a chain-like manner, thus creating an intracellular compass needle.
Every magnetosome contains a magnetic iron oxide core encased by a membrane. Apart from lipids, this membrane includes an array of proteins. At the University of Bayreuth, the microbiologists have successfully attached biochemically active functional groups— emerge from different foreign organisms—to these proteins.
The technique employed in this study communes at the stage of the bacterial genes that play a role in the biosynthesis of the membrane proteins. Such bacterial genes are coupled to foreign genes from other organisms that regulate the synthesis of the respective functional proteins.
Soon after the genes are re-incorporated into the genome, the reprogrammed bacteria create magnetosomes that exhibit these foreign proteins that were permanently fixed to the surface of the particles.
In the research, the membrane proteins were attached to four different functional groups or foreign proteins. Such functional groups include glucose oxidase, an enzyme released by a mold fungus, which already has been implemented biotechnologically, for instance, as a “sugar sensor” in diabetes diseases.
Additionally, a dye-producing enzyme produced by the bacterium Escherichia coli—whose activity can be easily determined—and a green fluorescent protein from a jellyfish were introduced on the magnetosomes’ surface.
An antibody fragment, obtained from a lama (Alpaca), represented the fourth functional group and this was utilized as a multipurpose connector. Therefore, the bacteria were genetically encoded with all these traits, including the exceptional magnetization of the magnetosomes.
Using this genetic strategy, we reprogrammed the bacteria to produce magnetosomes that glow green when irradiated with UV light and at the same time display novel biocatalytic functions. Various biochemical functions can be precisely installed on their surfaces. Thereby, magnetosomes from living bacteria are transformed into multifunctional nanoparticles with fascinating functions and properties.”
Dr Dirk Schüler, Study Lead and Professor, Department of Microbiology, University of Bayreuth
Dr. Schüler continued, “Moreover, the particles remain fully functional when they are isolated from the bacteria - which can be easily performed by taking advantage of their inherent magnetic properties.”
A genetic toolkit for applications in biomedicine and biotechnology
Functionalization of the magnetosomes is definitely not restricted to the functional groups that were adhered on the surface of the particles by the team of microbiologists at the University of Bayreuth. Rather, these proteins can be simply substituted by other functions, thereby offering a highly multipurpose platform.
Hence, genetic reprogramming opens up a wide spectrum to engineer the surface of magnetosomes. It offers the groundwork for a “genetic toolkit” that helps to create custom-made magnetic nanoparticles, integrating different useful properties and functions. Every particle measures 3–5 nm in size.
Our genetic engineering approach is highly selective and precise, compared to, for instance, chemical coupling techniques which are not as efficient and lack this high degree of control.”
Dr Frank Mickoleit, Study First Author and Microbiologist, University of Bayreuth
Dr. Mickoleit pointed out that the novel biomaterials offer a decisive benefit, “Previous studies show that the magnetic nanoparticles are likely not harmful to cell cultures. Good biocompatibility is an important prerequisite for the future application of the particles in biomedicine, for instance as contrast agents in magnetic imaging techniques or as magnetic sensors in diagnostics.”
“In the future, for example, similar particles might help to detect and destroy tumor cells. Bioreactor systems are another field of application. Magnetic nanoparticles equipped with tiny catalysts would be highly suitable for this purpose and enable complex biochemical processes,” Dr. Mickoleit added.
There is an enormous application potential for nanoparticles that display different functional groups on the surface, particularly in the fields of biotechnology and biomedicine. The magnetic bacteria now may serve as a platform for a versatile nano-toolkit, inspiring scientific creativity in the field of Synthetic Biology. It will initiate further interesting research approaches.”
Clarissa Lanzloth, BSc, Microbiologist, University of Bayreuth
Lanzloth was also involved in this latest research as co-author during the conclusion of her Master thesis in “Biochemistry and Molecular Biology” at the University of Bayreuth.
Source:
Journal reference:
Mickoleit, F., et al. (2020) A Versatile Toolkit for Controllable and Highly Selective Multifunctionalization of Bacterial Magnetic Nanoparticles. Small. doi.org/10.1002/smll.201906922.