Precise activation of selfish genetic elements called LINE-1 (L1) retrotransposons is essential to develop functional brain cells in a lab dish.
The results of a study by researchers at KAUST could enable more effective and safer regenerative therapies for the treatment of Parkinson’s disease and other brain conditions.
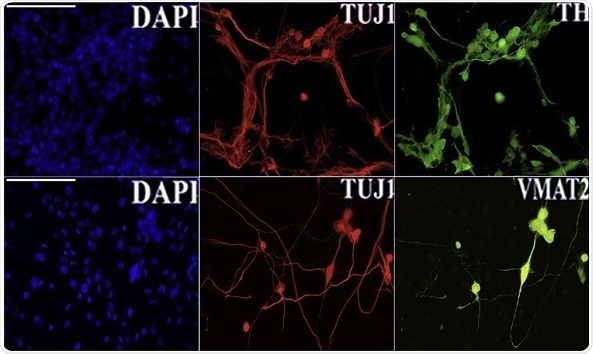
Reprogramming efficiency and cell conversion were confirmed by an immunofluorescent assay for principal neural commitment (TH, TUJ1, and VMAT2) and dopaminergic neuron markers (TH). Image credit: © 2020 Della Valle et al.
Hundreds and thousands of L1 elements can be found in the genomes of mice, humans, and other mammals. A majority of them are inactive, but a few exhibit the potential to duplicate themselves and jump into different segments of DNA. This leads to effects on gene regulation that can be both beneficial and harmful.
Occasionally, the jumping genes could even trigger the disease. However, in the early stages of brain development, L1 activity is inevitable for the proper formation of neurons, but why this is, is not clear.
To resolve this mystery, Valerio Orlando and his team resorted to using a cellular model of neuronal development. In collaboration with researchers from Italy, they designed skin cells derived from mouse embryos to express various reprogramming factors.
These reprogramming factors changed them into dopamine-synthesizing neurons, analogous to those found in the substantia nigra—a small structure found deep within the brain. L1 activation was observed by the researchers during this process.
The cells were treated with two types of drugs known to inhibit L1 dynamics. According to Francesco Della Valle, both treatments drastically affected the cell conversion efficiency, proving that “activation is required for successful reprogramming of skin cells into neuronal cells.” Della Valle is the first author of the study and a postdoc in Orlando’s lab group.
All the DNA within the cells was then sequenced both before and after their conversion to identify the segments into which the L1 elements had newly inserted themselves into the genome.
Insertional hotspots around the genes that take part in the neuron function and neuronal lineage commitment were identified. Thus, the DNA at these locations was not densely packed, which enabled higher levels of expression of the relevant gene.
Our work boosts the concept that repetitive elements play an important, unprecedented role in cell differentiation and tissue specific developmental programs.”
Francesco Della Valle, Study First Author and Postdoc, KAUST
The insights offered by this study were found to be very helpful to scientists developing various types of cell therapies for substituting the dopamine-generating neurons absent in people with Parkinson’s disease and associated disorders.
Aberrant L1 activity could threaten the viability or safety of any such product, while optimizing L1 function could enhance the manufacturing and consistency of this type of regenerative treatment.”
Francesco Della Valle, Study First Author, KAUST University
Source:
Journal reference:
Della Valle, F., et al. (2020) Transdifferentiation of Mouse Embryonic Fibroblasts into Dopaminergic Neurons Reactivates LINE-1 Repetitive Elements. Stem Cell Reports. doi.org/10.1016/j.stemcr.2019.12.002.