VRAC/LRRC8 chloride channels play a crucial role in the transport of neurotransmitters, amino acids, and cytostatics.
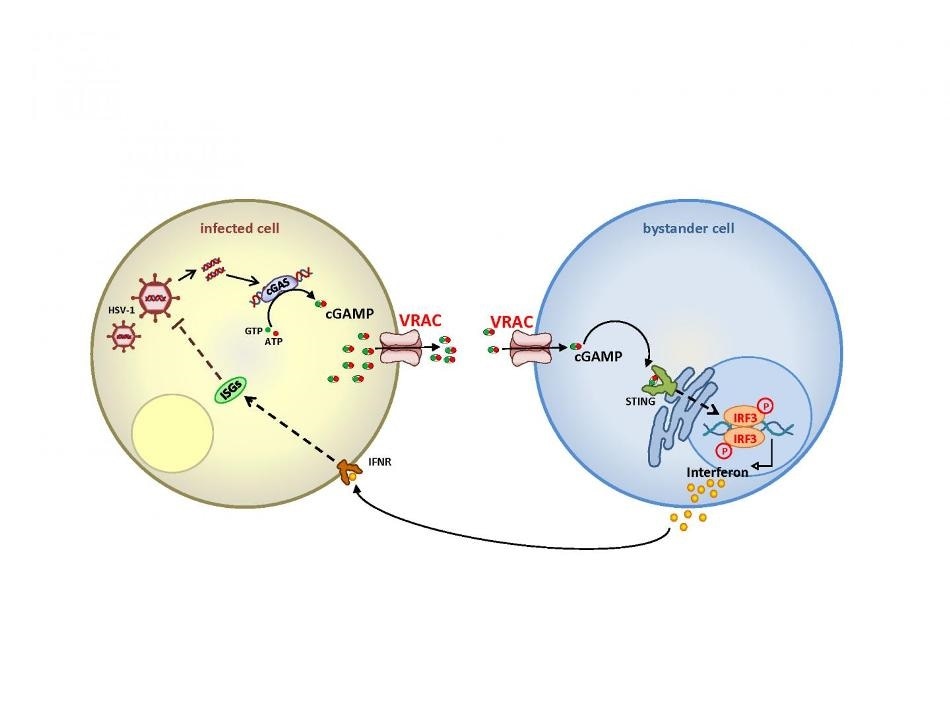
Upon infection of cells with a DNA virus (left), viral DNA binds to the enzyme cGAS which then synthesizes the messenger molecule cGAMP. The present work shows that cGAMP can leave the cell through the anion channel VRAC and diffuses to non-infected cells in the vicinity. After entering the cell—again through VRAC—it binds to a receptor called STING and stimulates indirectly the synthesis of interferon, which leaves the cell and suppresses, after binding to a receptor, the propagation of the virus (left cell). This provides a powerful amplification of the innate immune response against DNA viruses. Image Credit: Rosa Planells-Cases.
These channels are also capable of transporting cGAMP—the vital messenger substance—from one cell to another, thus reinforcing the immune response to infections caused by DNA viruses.
Now, this biological mechanism has been revealed by Professor Thomas Jentsch, who was the first to identify the LRRC8/VRAC channels. He currently works at the Leibniz Research Institute for Molecular Pharmacology (FMP) and the Berlin-based Max Delbrück Center for Molecular Medicine (MDC), along with collaborators from Shanghai headed by Professor Hui Xiao.
Considering that the cGAMP is invariably formed when DNA is detected by cells outside their nucleus, the latest finding could also prove to be highly significant for other types of pathologies like cancer. The study was recently published in Immunity, a scientific journal.
If DNA viruses, like herpes simplex, happen to infect human cells, this would be detected. Incidentally, the coronavirus, which is an RNA virus, is not a part of this group.
DNA has no place in the cell cytoplasm. Hence, if DNA is identified in the cytoplasm, messenger substances will form and start to trigger the alarm.
The foreign DNA attaches to the cGAS enzyme, which synthesizes cGAMP, that is, the “second messenger.” When this cGAMP binds to a receptor, known as STING, it stimulates a cellular signaling cascade that activates the production of various factors, including interferons, of the inherent immune system.
A mechanism like this has also been witnessed in cancer cells, where fragments of DNA are discharged from the nucleus into the cytoplasm, and also during certain bacterial infections.
cGAMP is a highly topical messenger substance
cGAMP studies have gained more attraction in the recent past, partly because it functions in the cell where it is synthesized and also travels to other types of cells. But researchers are still not clear how this is likely to occur.
In cells that make contact directly with one another, the cGAMP can travel via cell-connecting channels called “gap junctions.” However, what about the cells that are not there in the immediate surrounding?
Headed by Professor Hui Xiao from the Institut Pasteur Shanghai, scientists had suspected that the additional transport pathways should play a role and they eventually encountered the volume-regulated anion channel or VRAC for short.
This ion channel was detected in 2014 by Professor Thomas Jentsch and his research team, and simultaneously by Professor Zhaozhu Qiu who is currently at Johns Hopkins University and also contributed to the publication in the Immunity journal.
Using a wide range of techniques, the German-Chinese researchers jointly demonstrated that VRAC transports the cGAMP both into the recipient cell and out of the producing cell. As a result, the interferons are produced in the cells that are not infected, thus reinforcing their immune response.
We now know that VRAC definitely transports cGAMP. We didn’t know this function yet, but it fits well with our previous findings on VRAC, namely that it not only transports chloride, but also other small organic molecules, for instance neurotransmitters, amino acids and cytostatics.”
Thomas Jentsch, Professor, Department Physiology and Pathology of Ion Transport, Forschungsverbund Berlin
Jentsch continued, “The dependence of the cGAMP transport on the subunit LRRC8E—VRAC is always composed of several subunits— which we have now observed, agrees well with our earlier findings, which showed that this subunit supports the transport of glutamate, which is also negatively charged.”
Numerous cell culture experiments and electrophysiological techniques confirmed the uptake of the messenger substance by the VRAC.
For instance, in one such experiment, cells were first infected with a DNA virus and then separated from healthy cells with the help of a filter. While the infection caused by the virus could not be transmitted, the non-infected cells also displayed an interferon response.
Ultimately, experiments performed with knock-out rodents grown in Berlin and the ones which lacked LRRC8E—the VRAC subunit—provided conclusive proof that if the mice were infected with herpes viruses, a lower interferon release as well as a relatively higher viral load was noted when compared to that of the unmodified control animals.
This was exactly what we expected, because the messenger substance could no longer be transferred from infected cells to neighboring cells due to the absence of the channel. Since this transfer normally strengthens the immune response. The lack of cGAMP-transporting VRAC greatly reduces the defense mechanisms against such viruses.”
Thomas Jentsch, Professor, Department Physiology and Pathology of Ion Transport, Forschungsverbund Berlin
New strategies against DNA viruses and cancer
It is believed that the latest discovery of this novel role of VRAC in the body’s immune system against DNA viruses, a recent addition to the several crucial functions of VRAC, will garner relatively more attention to this ion channel.
According to scientists, VRAC may have an analogous role to play in cancer. Undeniably, other researchers have also demonstrated in animal models that the immune response against tumors is improved, when the transport of cGAMP from cancer cells to adjacent host cells improved, but how cGAMP is transported continues to be unclear.
Apart from gap junctions and VRAC, a folate transporter also delivers the cGAMP across the membrane, which was demonstrated the previous year. But VRAC is present in various types of cells and hence could play a bigger role.
In the days to come, it might provide a practical method to activate the VRAC to improve the immune response. The latest study has already described the potential ways to achieve this.
The field is incredibly hot, and our discovery offers completely new perspectives for both, infection research and cancer research.”
Thomas Jentsch, Professor, Department Physiology and Pathology of Ion Transport, Forschungsverbund Berlin
Source:
Journal reference:
Zhou, C. et al. (2020) Transfer of cGAMP into Bystander Cells via LRRC8 Volume-Regulated Anion Channels Augments STING-Mediated Interferon Responses and Anti-viral Immunity. Immunity. doi.org/10.1016/j.immuni.2020.03.016.