Lipids, also known as fats, have several crucial functions in the human body: They store energy, build membrane barriers, or serve as messengers, which mediate the growth of cells and also regulate the release of hormones.
A majority of lipids also act as biomarkers for severe diseases.
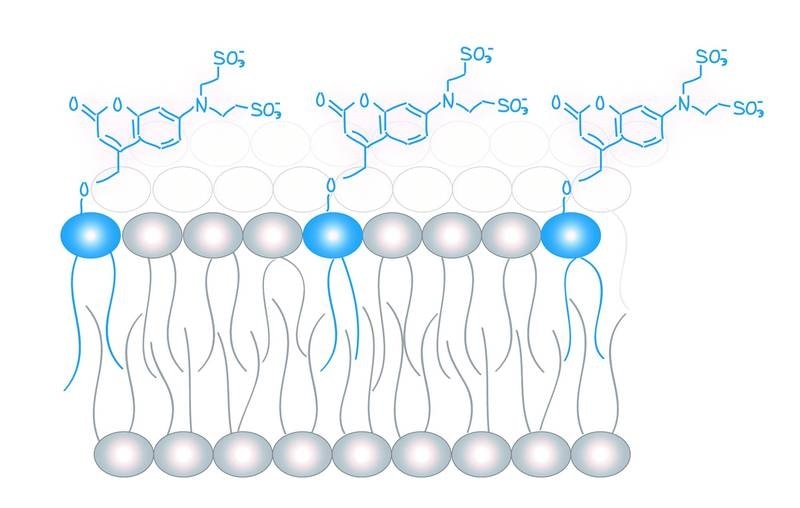
Molecular probes (in blue) for the analysis of lipid messengers. Image Credit: Copyright: Schuhmacher et al., MPI-CBG.
To date, examining the function of these molecules in living cells has been extremely difficult. Now, scientists from the Leibniz Research Institute for Molecular Pharmacology (FMP) in Berlin and the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden have created chemical tools that can be stimulated by light and utilized to impact the concentration of lipids in living cells.
This method could allow medical doctors to collaborate with biochemists to detect the type of molecules and their specific roles inside a cell. The research was published in the PNAS journal.
Each cell can produce an unlimited number of different lipids or fats. But how this chemical lipid diversity plays a role in transporting messages within the cell is not widely known—that is, the cell’s lipid code is still unfamiliar.
This can be mainly attributed to the dearth of methods to quantitatively analyze the function of lipids in living cells. An insight into the function of lipids is very crucial because they regulate protein function throughout the cell and play a role in bringing essential substances into the cell via the cell membrane.
In this process, only a minimal number of lipid classes on the interior of the cell membrane serve as messenger molecules, which is quite fascinating.
However, these lipid classes receive messages from an infinite number of different receptor proteins. Even now, it is not clear how this abundance of messages can still be effortlessly detected and transmitted.
The research teams, headed by André Nadler at the MPI-CBG and Alexander Walter at the FMP, in association with the TU Dresden, have created chemical tools to regulate lipid concentration in living cells.
Lipids are actually not individual molecular structures, but differ in tiny chemical details. For example, some have longer fatty acid chains and some have slightly shorter ones. Using sophisticated microscopy in living cells and mathematical modelling approaches, we were able to show that the cells are actually able to recognize these tiny changes through special effector proteins and thus possibly use them to transmit information.”
Milena Schuhmacher, Study Lead Author, Max Planck Institute of Molecular Cell Biology and Genetic
Schuhmacher continued, “It was important that we were able to control exactly how much of each individual lipid was involved.”
These results indicate the existence of a lipid code that cells use to re-encode information, detected on the outside of the cell, on the inner side of the cell.”
André Nadler, Max Planck Institute of Molecular Cell Biology and Genetics
Nadler also monitored the study.
The study results could allow lipid biochemists and membrane biophysicists to validate their results with quantitative data from living cells.
Clinicians could also benefit from our newly developed method. In diseases such as diabetes and high blood pressure, more lipids that act as biomarkers are found in the blood. This can be visualized with a lipid profile. With the help of our method, doctors could now see exactly what the lipids are doing in the body. That wasn't possible before.”
André Nadler, Max Planck Institute of Molecular Cell Biology and Genetics
Source:
Journal reference:
Schuhmacher, M., et al. (2020), Live-cell lipid biochemistry reveals a role of diacylglycerol side-chain composition for cellular lipid dynamics and protein affinities. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.1912684117.