Medical scientists from UNSW Sydney have successfully detected the interactions that occur between individual molecules located inside intact cells, thus achieving unparalleled resolution capabilities in single-molecule microscopy.
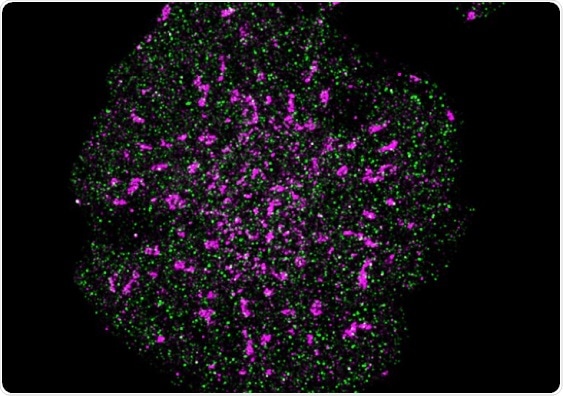
A T cell with precise localization of T cell receptors (pink) and CD45 phosphatase (green). Image Credit: Single Molecule Science.
The development of super-resolution fluorescence microscopy technology received the 2014 Nobel Prize in Chemistry. This technology allowed microscopists to get the first molecular view within the cells, a capability that has given new molecular viewpoints on complex biological processes and systems.
The limit of detection of single-molecule microscopes has now been shattered again, and the details of this study have been reported in the current issue of the Science Advances journal.
While super-resolution microscopy has already been used for observing and tracking individual molecules, the interactions between these kinds of molecules take place at a level of at least four times smaller than that resolved by prevalent single-molecule microscopes.
The reason why the localization precision of single-molecule microscopes is around 20-30 nanometers normally is because the microscope actually moves while we’re detecting that signal. This leads to an uncertainty. With the existing super-resolution instruments, we can’t tell whether or not one protein is bound to another protein because the distance between them is shorter than the uncertainty of their positions.”
Katharina Gaus, Scientia Professor and Research Team Leader, UNSW Sydney
Professor Gaus is also the Head of the EMBL Australia Node in Single Molecule Science at UNSW Medicine.
To overcome this issue, the researchers designed autonomous feedback loops within a single-molecule microscope that is capable of detecting and re-aligning the stage and the optical path.
“It doesn’t matter what you do to this microscope, it basically finds its way back with precision under a nanometer. It’s a smart microscope. It does all the things that an operator or a service engineer needs to do, and it does that 12 times per second,” added Professor Gaus.
Measuring the distance between proteins
Now, using the designs and techniques detailed in the study, the feedback system—developed by the UNSW researchers—can be used with present-day microscopes and offers the highest flexibility for preparing samples.
It’s a really simple and elegant solution to a major imaging problem. We just built a microscope within a microscope, and all it does is align the main microscope. That the solution we found is simple and practical is a real strength as it would allow easy cloning of the system, and rapid uptake of the new technology.”
Katharina Gaus, Scientia Professor and Research Team Leader, UNSW Sydney
The scientists demonstrated the usefulness of their ultra-precise feedback single-molecule microscope by conducting direct distance measurements between signaling proteins present in T cells.
In cellular immunology, one familiar hypothesis is that these immune cells continue to remain in a resting state, especially when the T cell receptor is close to another molecule that serves as a brake.
The high-precision microscope of the researchers revealed that both these signaling molecules are actually more isolated from one another in stimulated T cells, releasing the brake and turning on T cell receptor signaling.
Conventional microscopy techniques would not be able to accurately measure such a small change as the distance between these signaling molecules in resting T cells and in activated T cells only differed by 4–7 nanometres.”
Katharina Gaus, Scientia Professor and Research Team Leader, UNSW Sydney
“This also shows how sensitive these signaling machineries are to spatial segregation. In order to identify regulatory processes like these, we need to perform precise distance measurements, and that is what this microscope enables. These results illustrate the potential of this technology for discoveries that could not be made by any other means,” Professor Gaus concluded.
Source:
Journal reference:
Coelho, S., et al. (2020) Ultraprecise single-molecule localization microscopy enables in situ distance measurements in intact cells. Science Advances. doi.org/10.1126/sciadv.aay8271.