The information contained in DNA is crucial for life. Hence, the integrity of the DNA has to be maintained extremely well.
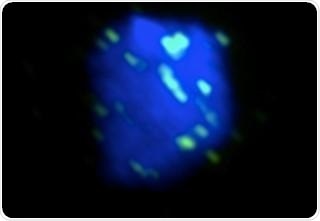
A yeast nucleus was irradiated with UV and imaged by fluorescent microscopy. Rad51 (green), the central protein in HR, localizes to DNA (blue) in order to repair the UV-induced damage. Image Credit: Tokyo Institute of Technology.
But DNA experiences damage at an alarming frequency because of the environmental factors and natural cellular processes. To offset this damage, cells have developed complex DNA repair processes to tackle this damage.
One of the mechanisms is called homologous recombination (HR) that employs the protein referred to as Rad51.
Many proteins function together to help this Rad51 protein, even though the latter plays the main role in HR. These helper proteins often create distinct groups containing multiple subunits.
Overview of research achievement
To stimulate the Rad51 protein, the helper proteins initially had to adhere to this protein. To demonstrate how a helper protein called Swi5-Sfr1 binds to the Rad51 protein, the YCU team used nuclear magnetic resonance spectroscopy.
This allowed the team to discover that a pair of regions inside the Sfr1 plays a role in anchoring the Swi5-Sfr1 to the Rad51 protein.
The Tokyo Institute of Technology (Tokyo Tech) team then combined the purified DNA and purified proteins in a test tube and effectively illustrated that mutations occurring in these two regions affected the activation of the Rad51 protein by Swi5-Sfr1.
The scientists reasoned that yeast cells comprising mutations in both these regions of Sfr1 would not be able to repair the damage caused to DNA, but at the same time, they were surprised to note this was not the case.
The researchers conceptualized that the DNA repair process is probably rescued by the protein present in the cell but absent in the test tube, which just comprises meticulously chosen purified materials.
The two HR sub-pathways in yeast were previously disclosed by the laboratory of senior author Professor Hiroshi Iwasaki from Tokyo Tech. Here, one HR sub-pathway depends on the Swi5-Sfr1, while the other relies on the Rad51-related helper proteins
To find out if this other sub-pathway was counteracting the function of mutant Swi5-Sfr1, the researchers created yeast cells that lacked the Rad51-associated helper proteins and subsequently re-analyzed whether DNA repair could be promoted by the mutant Swi5-Sfr1.
The mutant Swi5-Sfr1 did not stimulate the Rad51 protein and the cells were not able to fix their DNA. This indicates that the role of the Swi5-Sfr1 helper protein in stimulating the Rad51 protein is promoted by Rad51-associated helper proteins.
The lack of Rad51activation by the Swi5-Sfr1 mutant and its inability to fix their DNA is indeed noteworthy. This biological mechanism suggested that the role of the Swi5-Sfr1 helper protein in stimulating the Rad51 protein is supported by the Rad51-related helper proteins.
Future development
Now, the researchers have suggested that instead of working independently of one another as was believed in the past, the Swi5-Sfr1 and the Rad51-related helper proteins combine to trigger the Rad51 protein.
We have seen this collaborative effort between the helper proteins to repair DNA in living cells. Now we want to reconstitute the activation of Rad51 in a test tube with both Swi5-Sfr1 and the Rad51-related helper proteins. We believe this approach will be effective in uncovering the finer details involved in this interplay.”
Dr Bilge Argunhan, Study Lead Author, Tokyo Institute of Technology
Dr. Argunhan is a specially appointed assistant professor in Professor Hiroshi Iwasaki’s laboratory.
Several cancers and other human diseases are linked to defects in HR. Considering that the underlying mechanisms of HR are evolutionarily preserved from yeast to humans, the authors have planned to integrate the robust genetics that can be achieved in yeast with biochemical methods to achieve further understanding of DNA repair that may be applicable to figure out the human disease.
Source:
Journal reference:
Argunhan, B., et al. (2020) Cooperative interactions facilitate stimulation of Rad51 by the Swi5-Sfr1 auxiliary factor complex. eLife. doi.org/10.7554/eLife.52566.