Cancer can sometimes remain dormant, but it usually metastasizes and spreads to new sites in the body. For a long time, it has been suspected that this possibly devastating turn of effects is driven by genetic mutations emerging within the tumor cells.
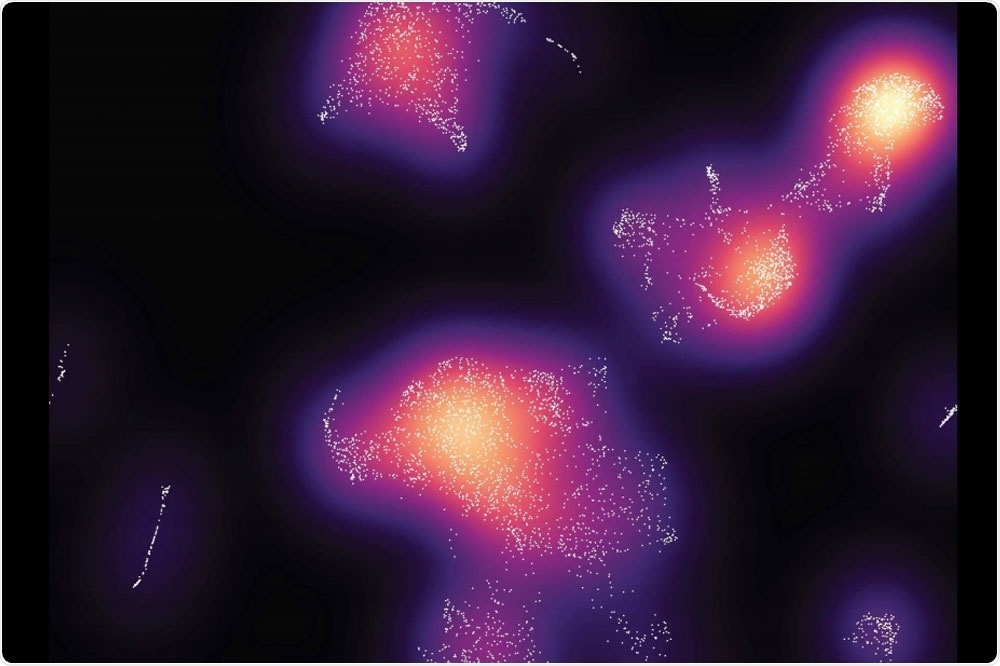
When researchers analyzed the immune cells in the tumors and grouped them by type (above), they found more cancer-fighting cells in mice with ApoE4. Image Credit: Elizabeth and Vincent Meyer Laboratory of Systems Cancer Biology at The Rockefeller University.
Now, for the first time, scientists have demonstrated that metastasis can be promoted by a person’s own pre-existing genetics.
According to the latest study published in the Nature Medicine journal on May 25th, 2020, the variations in a single gene, carried inside someone’s genome from birth, can modify the progression of a type of skin cancer called melanoma.
The scientists suspected that these inherited variations might also have the same impact on other types of cancer cells.
Patients often ask ‘Why am I so unlucky? Why did my cancer spread?’ As doctors, we never had an answer. This research provides an explanation.”
Sohail Tavazoie, Lead Investigator and Leon Hess Professor, Rockefeller University
Tavazoie, who is also a senior attending physician at the Rockefeller University, added that this finding may redefine the way researchers think about cancer metastasis and result in a deeper understanding of patients’ risks to inform treatment decisions
The mystery of metastasis
Metastasis takes place when cancer cells establish new tumors in some other locations by escaping from the original tissue. This is a phenomenon that is responsible for the majority of cancer deaths. Researchers have suspected that cancer cells, which originally arise because of mutations in the interior of normal cells, gained their migrating ability by tracking additional mutations.
However, after many years of searching, scientists have not yet found such a genetic variation that could be known to promote metastasis.
Earlier research performed in Tavazoie’s lab has detected a gene known as APOE, located in the DNA of all the cells of the body before the development of any cancer, that can affect the spread of melanoma.
The APOE gene produces a protein that seems to disrupt several processes used by cancer cells to metastasize, like the formation of blood vessels, growing deeper into the healthy tissue, and tolerating the assault from cancer-fighting immune cells.
However, humans carry any one of the three different versions of ApoE, ApoE2, ApoE3, and ApoE4. Benjamin Ostendorf, a physician-scientist in Tavazoie’s lab, hypothesized that such variants may explain why melanoma develops differently in different individuals.
In experiments conducted with mice having one of each of the gene versions, Ostendorf and collaborators discovered that tumors in mice with ApoE4 grew the smallest and proliferated the least.
Upon closer analysis, it was found that ApoE4 happens to be the most effective version of ApoE in terms of improving the immune response to cancer cells. In comparison to animals with other variants, the mice carrying the ApoE4 gene displayed a greater abundance of tumor-fighting T cells that were recruited into the melanoma tumor and also reduced the blood vessels.
Ostendorf stated, “We think that a major impact of the variations in ApoE arises from differences in how they modulate the immune system’s attack.”
Toward better treatment
Genetic information from over 300 human melanoma patients mirrored the mouse experiments. On average, persons who had the ApoE4 gene survived the longest, whereas those with the ApoE2 gene lived the shortest. This link to outcomes implies that doctors could observe the genetics of the patients to examine the risk of their cancer development.
This could also impact the treatment course. At times, melanoma patients are given therapy to promote their own immune system to fight cancer in a better way.
The researchers’ analysis of information obtained from such patients and also from the mice experiments proved that those with the ApoE4 gene respond best to immune-boosting treatments.
Similarly, the scientists demonstrated that an experimental compound that boosts the production of ApoE, RGX-104, was effective at aiding mice with the ApoE4 gene to fight against tumors. RGX-104 is presently in clinical trials. Tavazoie is a scientific co-founder of Rgenix, the firm that developed RGX-104.
According to Tavazoie, more research is required to determine the best way to improve the treatments for patients who have the other ApoE variants. For example, ApoE2 was linked to an increased risk of metastasis.
So far, scientists’ evidence suggests that the metastasis-suppressing ability of the ApoE3 falls between that of the other two, that is, ApoE2 and ApoE4.
We need to find those patients whose genetics put them at risk for poor survival and determine what therapies work best for them.”
Sohail Tavazoie, Lead Investigator and Leon Hess Professor, Rockefeller University
The implications may extend further than cancer. Other studies have demonstrated that ApoE variations cause Alzheimer’s disease, the ApoE4 version aggravates the risk of this neurodegenerative disorder, contrary to its suppression of cancer progression.
It’s not quite clear what ApoE does in Alzheimer’s, but we believe our work in cancer can inform our understanding of this disease as well.”
Sohail Tavazoie, Lead Investigator and Leon Hess Professor, Rockefeller University
Tavazoie lab, which generally focused on cancer, has started to investigate the association with the neurodegenerative disorder.
Source:
Journal reference:
Ostendorf, B. N., et al. (2020) Common germline variants of the human APOE gene modulate melanoma progression and survival. Nature Medicine. doi.org/10.1038/s41591-020-0879-3.