For the first time, researchers from Van Andel Institute have explained the near-atomic level structure of a molecular route that has crucial roles to play in the regulation of blood pressure, cell death, inflammation, and human development. The results were recently published in the Nature journal.
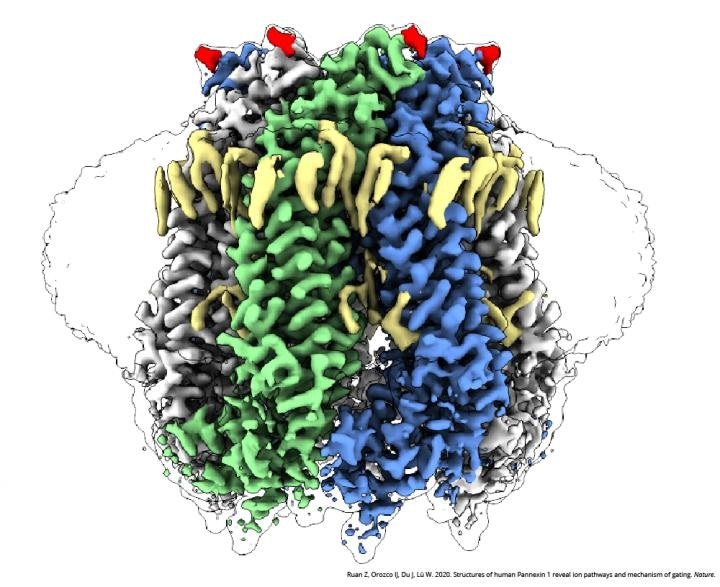
A cryo-EM image of PANX1. Image Credit: Courtesy of Dr Wei Lu and Dr Juan Du, Van Andel Institute.
The pannexin1 channel, or PANX1, is vitally important for maintaining normal, healthy function and, as such, is a major target for treating a host of diseases. Our new images provide a blueprint for drug development and also answer longstanding questions about how PANX1 works.”
Wei Lü, PhD, Study Co-Corresponding Author and Assistant Professor, Structural Biology Program, Van Andel Institute
PANX1 is a type of protein that is found in tissues across the body, where it creates channels that permit the passage of molecules, like the cellular fuel ATP, together with relatively smaller ions. Drugs that disrupt the PANX1 protein have been demonstrated as potential therapies for several diseases, such as cancer and cardiovascular diseases. To date, not much was known about the exact interaction of these agents with the PANX1 protein.
The research revealed vital changes that occur in the PANX1 protein during typical conditions against times when the cells are experiencing apoptosis—a process that recycles dead and damaged cells. Such variations cause ions and molecules to be guided through different sites of the channel and to find out whether a large pore is closed or opened.
The pore is blocked under regular conditions, and only the small ions are permitted to enter seven narrow side tunnels in the PANX1 protein. But at the time of apoptosis, the pore is opened and ATP is discharged, which, in turn, transmits a signal that triggers cellular recycling.
Most significantly, the researchers also identified that the function of a common medication that blocks PANX1, carbenoxolone, is to prevent other molecules from intruding into the channel. Carbenoxolone, which is used for ulcer treatment, is being analyzed as a therapy for various types of cancers.
These insights will be impactful beyond our understanding of PANX1—they also shed light on how other similar large-pore channels function.”
Juan Du, PhD, Study Co-Corresponding Author and Assistant Professor, Structural Biology Program, Van Andel Institute
Apart from Du and Lü, other study authors include lead author Zheng Ruan, PhD, and Ian J. Orozco, PhD from Van Andel Institute. The state-of-the-art David Van Andel Advanced Cryo-Electron Microscopy Suite at Van Andel Institute facilitated the findings, allowing researchers to visualize some of life’s smallest components in vivid detail.
The Titan Krios is the most powerful microscope of Van Andel Institute and can visualize molecules 1/10,000th the thickness of a human hair. The high-performance computing group at Van Andel Institute also extended support for this research.
PANX1 Courtesy of Dr. Wei Lu and Dr. Juan Du - Van Andel Institute
The pannexin1 channel, or PANX1, plays critical roles in human development, blood pressure regulation, inflammation, and cell death. Its structure has been visualized at the near-atomic level using cryo-electron microscopy. Video Credit: Courtesy of Dr Wei Lu and Dr Juan Du, Van Andel Institute
Source:
Journal reference:
Ruan, Z., et al. (2020) Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature. doi.org/10.1038/s41586-020-2357-y.