At the Stowers Institute for Medical Research, researchers have demonstrated that a dysfunctional placenta can play a formerly unknown role during the earliest phases of development in mouse models of Cornelia de Lange syndrome.
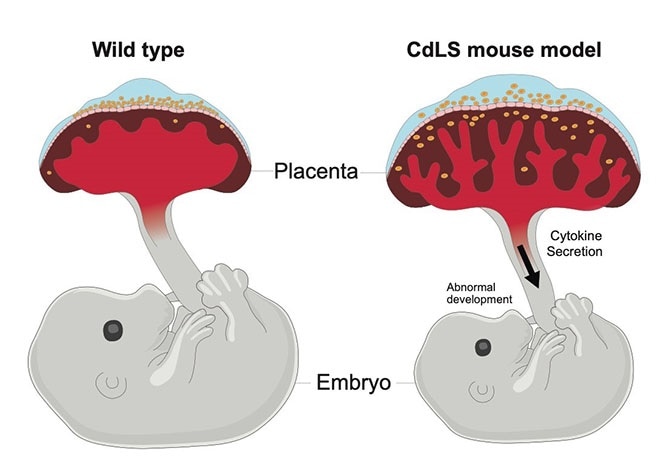
The placenta is essential for embryo development and acts as an interface between the embryo and mother for the exchange of gas, nutrient, and waste products. Mutations that affect cohesin genes cause the human developmental disorder Cornelia de Lange syndrome (CdLS). Mouse models of CdLS show placental abnormalities such as increased placental size and activated cytokine signaling, which leads to embryo growth retardation. Image Credit: Mark M. Miller, Stowers Institute for Medical Research.
Individuals suffering from this rare genetic disorder frequently harbor mutations in cohesins—ring-like proteins that aid DNA to arrange and repair it.
The scientists observed that mice with cohesin mutations had placentas that built up damage to their DNA assumed a permanent growth-arrested state called senescence, and released pro-inflammatory cytokines that impacted the growth of the embryos.
The results, published online in the Developmental Cell journal on June 16th, 2020, indicate that targeting cytokine signaling could offer a way to safeguard the health of the placenta and support healthy pregnancies.
The discovery that DNA damage activates senescence in the placenta has huge implications, In terms of potential relevance to human health, our research suggests that this could be one mechanism through which things like smoking, which can cause DNA damage and intrauterine growth restriction, might be acting.”
Jennifer L. Gerton, PhD, Study Lead Author and Investigator, Stowers Institute for Medical Research
It has been estimated that intrauterine growth restriction affects 1 in 20 newborns. In this condition, a baby is smaller than predicted because it is not developing at normal speed within the womb. Cornelia de Lange syndrome, a rare medical disorder that affects 1 in 10,000 infants, is one of the best examples of this stunted growth.
Individuals born with this disorder can have abnormalities of bones in the hands, fingers, and arms, intellectual disability, and short stature.
Cornelia de Lange syndrome is induced by mutations in genes that influence a complex of proteins known as cohesins that surround DNA and fold it into loops. For a long time, researchers have assumed that the syndrome appears when defects in cohesin complexes disrupt the organization of DNA, disturbing the way genes are switched on and off during development. But Gerton with her collaborators suspected that was just one part of the story.
Earlier studies showed that women who were carrying babies affected by Cornelia de Lange syndrome had reduced levels of a protein known as pregnancy-associated plasma protein-A (PAPP-A). Since this protein is secreted by the placenta, the outcomes indicated that something may be amiss with this crucial yet understudied organ.
Vijay Pratap Singh, PhD, a postdoc in the Gerton laboratory, decided to explore what was occurring to the placenta in mouse models of Cornelia de Lange syndrome. He subsequently detected persistent damage to their genomes, emphasizing a significant yet unexplored role of cohesins in repairing DNA damage. Such maintenance problems sent the placenta into a premature state of senescence, where its cells no longer divide.
Singh revealed that as the cells of the placenta moved into senescence, they started to secrete chemicals called cytokines that trigger the inflammatory response, similar to flares to signal danger. These cytokines collected in the embryonic mice, impacting their growth and health.
There is an old saying that during pregnancy, any kind of stress can affect the baby’s growth. Here, using mouse models, we have shown on a molecular level how DNA damage can affect embryonic growth through cytokine signaling.”
Vijay Pratap Singh, PhD, Study First Author and Postdoc, Gerton laboratory
To additionally investigate the role of the placenta in Cornelia de Lange syndrome, Singh analyzed whether a normal mouse placenta could decrease the growth defects of a Cornelia de Lange mouse model embryo to investigate the function of the placenta in Cornelia de Lange syndrome.
Surprisingly, he found that the mouse model embryos with Cornelia de Lange and supported by normal mouse placentas ensured better and were healthier when compared to those fed by affected placentas.
Going forward, the scientists have planned to test whether they can also produce better results in mouse models of the syndrome with small-molecule drugs that inhibit pro-inflammatory cytokines in the placenta. According to Gerton, such anti-inflammatory drugs may one day enhance pregnancy results, but more studies are required in this area.
Sean McKinney, Ph.D., head of the Stowers Microscopy Center, was also the study’s co-author. The study was financially supported by the Stowers Institute for Medical Research and the March of Dimes.
Source:
Journal reference:
Singh, V. P., et al. (2020) Persistent DNA Damage and Senescence in the Placenta Impacts Developmental Outcomes of Embryos. Developmental Cell. doi.org/10.1016/j.devcel.2020.05.025.