When the body continues to perform its daily biological processes, the molecules, known as neurotransmitters, regulate the level of electrical activity inside the brain.
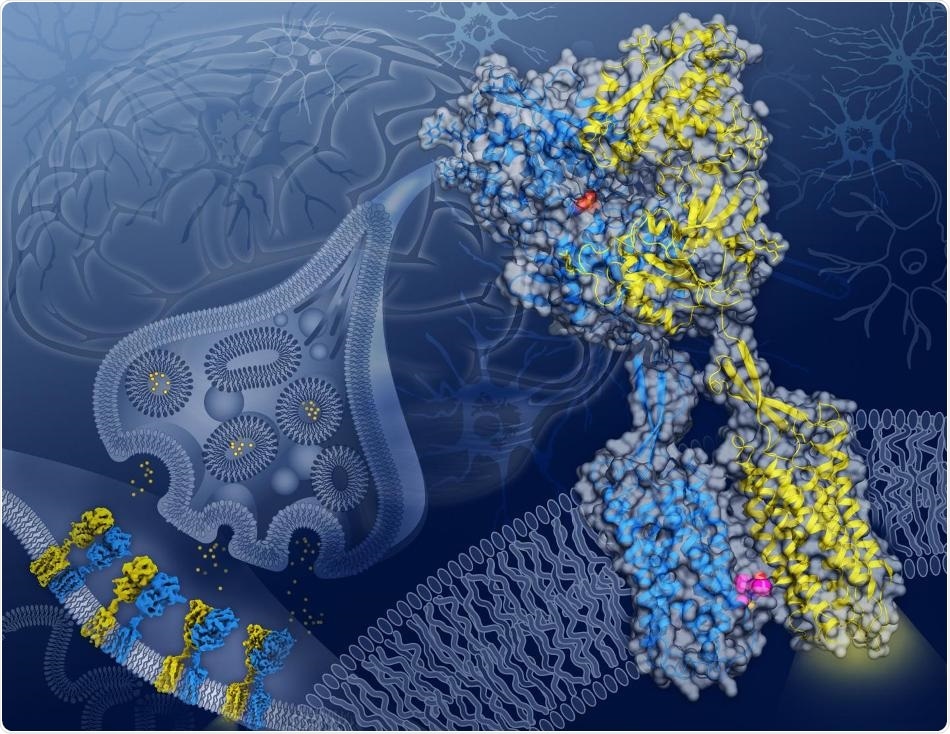
In this artist’s depiction, cryo-EM structures of GABAB captured in four different conformations along its activation transition appear on a neuronal synapse (background) while the structure of the GABAB receptor in the active state with agonist (orange spheres) bound in GB1 (blue) and a PAM (magenta spheres) bound on the transmembrane interface between GB1 and GB2 (yellow) appears in close-up. Image Credit: Illustration by Yekaterina Kadyshevskaya/Bridge Institute at the USC Michelson Center for Convergent Bioscience.
These neurotransmitters, which interact with protein receptors huddled in the membrane that constitutes the outer border of a neuron, open and closes the portals that regulate the passage of ions in and out of the cell.
Gamma-aminobutyric acid, or GABA for short, dominates as one of the most significant inhibitory neurotransmitters in the brain. The main role of GABA is to calm the activity of the brain, decreasing the number of signals firing in the brain to complement the activity of other neurotransmitters that speed up this organ’s activity. (It is even sold as a nutritional supplement to support calmness and enhance sleep.)
When GABA is incapable of inhibiting brain signaling, the imbalance in activity can result in several health problems, including muscle spasms, increased pain, anxiety or mood disorders, and, in extreme cases, even epilepsy.
In a study published in the Nature journal on June 17th, 2020, researchers from Dornsife College of Letters, Arts and Sciences at the University of Southern California (USC) and the Bridge Institute at the USC Michelson Center for Convergent Bioscience identified the mode of interaction between GABA and a major protein receptor known GABAB.
Performed in association with scientists at Stanford University, the Stanford Linear Accelerator Center, and the Université de Montpellier based in France, the research clearly shows how GABA modifies the shape of GABAB and exposes a clear target for novel drugs.
Ultra-high-definition views of a shape-shifter
The scientists employed cryo-electron microscopy, or cryo-EM, to capture comprehensive images of the GABAB receptor protein that were never seen before. This receptor protein twists and contorts when interacting with GABA.
The GABAB receptor protein, which settles over the cellular membrane of neurons, is made up of two subunits with analogous shapes: GB1, which identifies GABA, and GB2, which transmits the signal from GB1 into the interior of the cell.
The images captured with cryo-EM offer blueprints of how, as GABA communicates with GB1, a ripple effect spreads across the whole protein until a cavity opens in the section of the GB2 subunit that faces within the cell. Once exposed, this cavity in GB2 can communicate with and trigger proteins inside the cell that regulate the activity of the neuron.
The three-dimensional (3D) structural images may lead to better treatments for neurological conditions.
These blueprints can be used to design new therapeutic drugs that could affect different conformational states and thus act more precisely.”
Vadim Cherezov, Study Corresponding Author, Professor of Chemistry, Dornsife College of Letters, Arts and Sciences, University of Southern California
More significantly, the images also exposed an important location on the GABAB receptor protein, where both GB1 and GB2 meet inside the cell membrane upon activation of the receptor.
This GB1-GB2 interface, which was unknown before, is a major target for a type of drug known as a positive allosteric modulator, or PAM for short.
According to Cherezov, PAMs could help develop advanced therapeutic drugs since they do not substitute the action of naturally occurring molecules, like GABA, but instead fine-tune the behavior of the receptor, resulting in fewer side-effects.
There are many therapeutic areas in which targeting the GABAB receptor protein could be beneficial; however, such direct interventions may come with significant side-effects. Developments of PAMs facilitated by our structure images could lead to a new generation of safer drugs targeting this receptor.”
Vadim Cherezov, Study Corresponding Author and Professor of Chemistry, Dornsife College of Letters, Arts and Sciences, University of Southern California
Source:
Journal reference:
Shaye, H., et al. (2020) Structural basis of the activation of a metabotropic GABA receptor. Nature. doi.org/10.1038/s41586-020-2408-4.