The quest to detect all kinds of proteins that constitute the sarcomere, the fundamental contractile unit of muscle cells, led to a surprising revelation, offering experimental proof that helps describe an underlying mystery about the working of muscles.
The study was published in the Nature Communications journal.
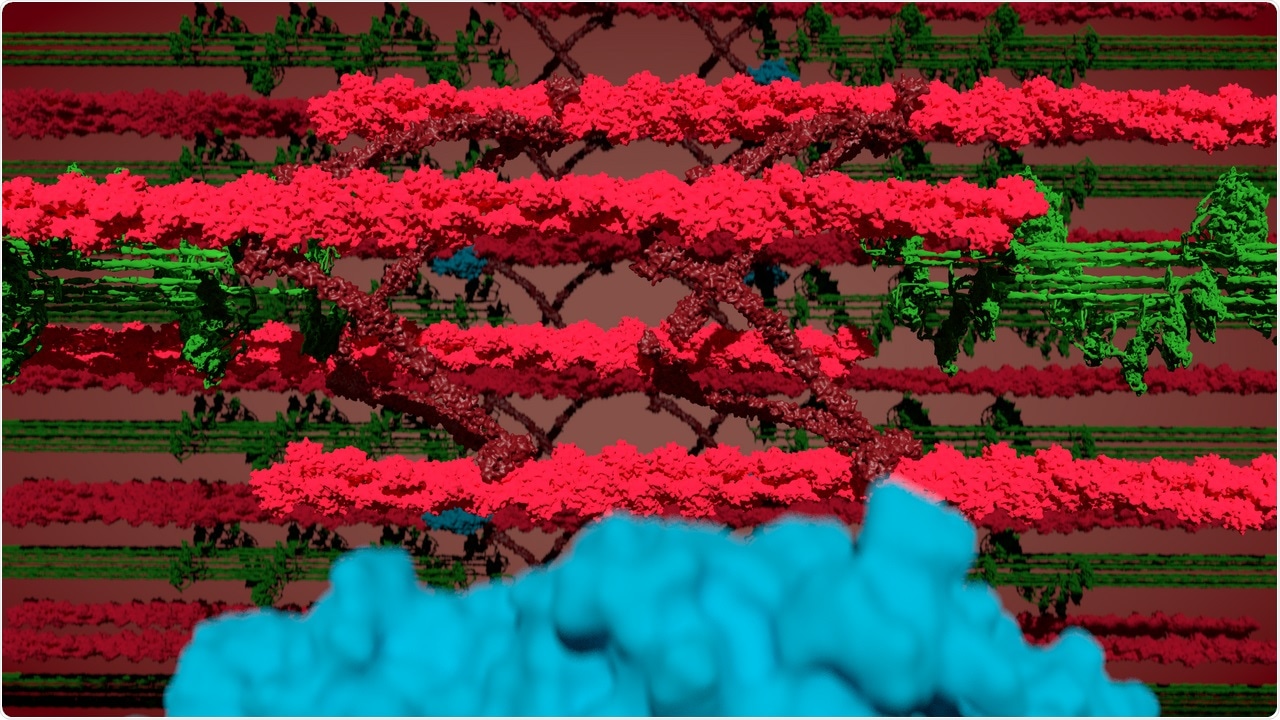
Watching the sarcomeres contract—collage of myosin (green), actin, and the Z-disk (red) and BioID (blue). Image Credit: © Jacobo Lopez Carballo, Gotthardt Lab, MDC.
At the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), scientists have created a mouse model that allows them to peer inside a working muscle and detect the proteins that enable the sarcomere to relax, contract, convey its energy requirements, and acclimatize to exercise.
In particular, the researchers were able to map the proteins present in the defined sub-regions of the sarcomere, beginning from the “Z-disk,” the margin between adjacent sarcomeres. This by itself is a major step forward in the research of striated muscle.
The researchers made a surprising discovery during the process: myosin, one among the three primary proteins that constitute striated muscle fibers, seems to penetrate the Z-disk.
Models of how actin, myosin, and titin—the elastic scaffold protein—work collectively have mostly overlooked the possibility that filaments of myosin enter the Z- disk structure. German scientists have now only theorized that they indeed do but there was no experimental proof to validate the model, to date.
This is going to be unexpected even for myosin researchers. It gets to the very basics of how muscles generate force.”
Michael Gotthardt, Study Lead and Professor, Max Delbrück Center for Molecular Medicine in the Helmholtz Association
Gotthardt also heads the Neuromuscular and Cardiovascular Cell Biology Lab at MDC.
Who Is there?
Gotthardts’ research group, including the study’s first authors Dr. Franziska Rudolph, Dr. Claudia Fink, as well as collaborators from the MDC and the University of Göttingen, never embarked to verify this concept.
The primary aim of the researchers was to detect the proteins in and around the Z-disk. To achieve this, the team created a mouse model with an artificial enzyme, known as BioID, embedded into the large protein titin. The Titin-BioID subsequently tagged the proteins close to the Z-disk.
Sarcomeres are essentially small molecular machines, packed with tightly interacting proteins. To date, researchers were not able to isolate proteins specific to the different sub-regions, particularly in live and functioning muscles.
Philipp Mertins, who also heads the Proteomics Lab at MDC, stated, “Titin-BioID probes specific regions of the sarcomere structure in vivo. This has not been possible before.”
The researchers were the first to utilize BioID in live animals kept under physiological conditions and discovered as many as 450 proteins linked to the sarcomere, out of which around 50% were already established. The team found prominent variations between skeletal and heart muscles, and neonatal versus adult mice, which are associated with the structure, signaling, and metabolism of the sarcomere.
Such variations reflect the requirement for adult tissue to enhance energy production and performance against growth and remodeling in neonatal tissues.
Gotthardt added, “We wanted to know who’s there, know who the players are. Most were expected, validating our approach.”
The surprise
However, the researchers did not expect to see myosin in the Z-disk. This myosin is embedded at the opposite location of the sarcomere. When a muscle is activated to move, myosin walks along actin and brings the adjacent Z-disks closer together.
This sliding action of actin and myosin filaments generates a force that allows the persons’ heart to pump blood or their skeletal muscle to lift an object or maintain posture.
This supposed “sliding filament model” of the sarcomere explains the generation of force and helps describe the relationship between force and the sarcomere length. But new models find it hard to predict the behavior of sarcomeres that have fully contracted.
According to those models, myosin does not penetrate the Z-disk on its walk along actin. A few clues were there that perhaps it keeps going.
But we didn’t know if what we were seeing in stained tissue samples was an artefact or real life. With BioID we can sit at the Z-disc and watch myosin pass by.”
Michael Gotthardt, Study Lead and Professor, Max Delbrück Center for Molecular Medicine in the Helmholtz Association
Gotthardt conceded with the proposed concept that myosin penetrating the Z-disk can dampen or restrict the contraction. This may help solve the current problems faced by researchers when it comes to calculating the amount of force exerted by a muscle fiber with respect to its length and results in a more improved model of the sarcomere, and perhaps protect the muscles from extreme contraction.
Why it matters
Having an insight into the extension and contraction of muscle fibers on the molecular level under regular conditions is significant, as this would allow scientists to know what is going wrong when muscles are diseased, damaged, or atrophy with age.
Detecting the type of proteins that are responsible for causing the problems may help identify new treatment targets for patients suffering from skeletal muscle disorders or heart disease.
Gotthardt and his research team have next planned to use BioID to analyze animals with various pathologies, for instance, to observe the proteins involved in muscle atrophy.
Maybe a protein that is not normally there goes into the sarcomere, and it is part of the pathology. We can find it with BioID.”
Michael Gotthardt, Study Lead and Professor, Max Delbrück Center for Molecular Medicine in the Helmholtz Association
Source:
Journal reference:
Rudolph, F., et al. (2020) Deconstructing sarcomeric structure–function relations in titin-BioID knock-in mice. Nature Communications. doi.org/10.1038/s41467-020-16929-8.