Researchers have performed a new study that exposes complex details regarding the biology of the huntingtin protein (HTT), which causes Huntington’s disease.
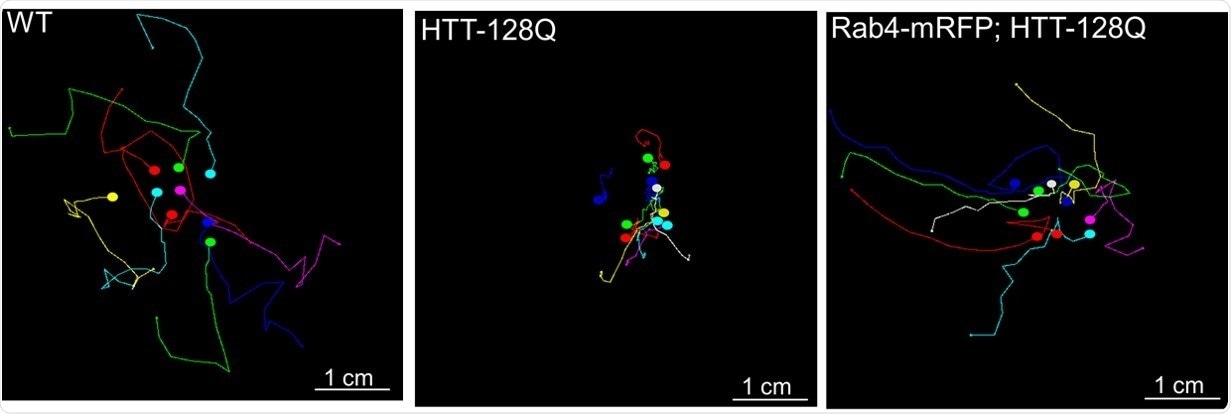
Colored lines show the paths of crawling fruit fly larvae. Larvae with a mutant form of the human huntingtin protein that causes Huntington’s disease crawled poorly (center) compared with normal larvae (left). But when larvae carrying the Huntington’s disease protein also produced extra quantities of the Rab4 protein, crawling improved, though not completely (right). Image Credit: White, J.A., Krzystek, T.J., Hoffmar-Glennon, H. et al., Acta Neuropathologica Communciations, July 2020. The image is adapted from a graphic in the journal article, which is distributed under Creative Commons Attribution 4.0 International License, https://creativecommons.org/licenses/by/4.0/.
The study targeted axonal transport—the route through which essential materials move along pathways known as axons within neurons, or nerve cells.
The researchers observed that at times, HTT travels along these pathways in cellular vehicles—also known as vesicles—that also carry freight, like a protein known as Rab4. The researchers concluded that other materials that may exist in these cargoes include proteins that are crucial to the function and health of nerve cells.
The discoveries also point to a possible treatment avenue: In the case of fruit fly larvae, a mutated version of HTT that is responsible for causing Huntington’s disease interrupted the regular movement of vesicles that hold Rab4 and HTT. This resulted in problems like defects in a section of the nerve cell, known as the synapse; reduced lifespan; and crawling difficulties.
However, when the researchers engineered larvae carrying the HTT mutation to also create extra Rab4, a few of these symptoms, such as abnormalities in crawling, synapses, and lifespan, became less pronounced.
When it comes to finding therapies for neurological diseases, a lot of the research focuses on the pathology and aggregation of proteins, but that may not be the actual cause. We want to try to figure out what might be going on earlier. We want to come in from a different angle and say, ‘OK, are huntingtin and Rab4 normally present together in healthy neurons? And then, what are they doing in the context of a disease state?’”
Shermali Gunawardena, PhD, Associate Professor, Department of Biological Sciences, College of Arts and Sciences, University at Buffalo
“This research helps shed light on the normal function of huntingtin and how it is altered in disease. Based on what we’re seeing, HTT seems to be important for the transport of a particular type of vesicle known as endosomes within neurons,” stated Joseph A. White II, the study’s co-first author and currently a postdoctoral researcher at Duke University.
White II continued, “We believe this presents a potential avenue for therapeutics aimed at improving endosomal transport in Huntington’s disease patients.” White II was a PhD Graduate at the Department of Biological Sciences in the College of Arts and Sciences at the University at Buffalo.
Gunawardena is the study’s corresponding author and headed the research with first authors White II and Thomas J. Krzystek, a PhD student in the Department of Biological Sciences at the University at Buffalo.
Recently published on in the Acta Neuropathologica Communications journal on July 1st, 2020, the study builds on previous research performed in Gunawardena’s laboratory. Earlier, Gunawardena’s team demonstrated that the mutated form of HTT can also disrupt the movement of certain other Rab proteins.
According to Gunawardena, the findings indicate that promising treatments targeting axonal pathways may also have to be multifaceted, involving several drugs that target a different set of problems.
The idea behind our work is to tease out these details about what’s going on inside neurons. It’s really difficult to isolate what’s going on inside the organism. But from what we have done in the lab, we can say now that huntingtin is in these different, specific kinds of cargoes. I think the bottom line is that this protein is doing a lot of different things.”
Shermali Gunawardena, PhD, Associate Professor, Department of Biological Sciences, College of Arts and Sciences, University at Buffalo
Model organisms, like the fruit fly, are excellent tools we can use to quickly investigate a variety of proteins and pathways related to human diseases. Our study highlights the advantage of using these simpler organisms to discover new information that we can then test directly in more complex systems.”
Thomas J. Krzystek, PhD Student, Department of Biological Sciences, College of Arts and Sciences, University at Buffalo
“Through this, we learned about what huntingtin might be doing with Rab4 in healthy neurons, and how this role may become disrupted in Huntington’s disease,” Krzystek concluded.
Source:
Journal reference:
White II, J. A., et al. (2020) Excess Rab4 rescues synaptic and behavioral dysfunction caused by defective HTT-Rab4 axonal transport in Huntington’s disease. Acta Neuropathologica Communications. doi.org/10.1186/s40478-020-00964-z.