Scientists from the Korea Advanced Institute of Science and Technology (KAIST) have successfully dialed up and down the lifespan of creatures by changing the activity of proteins present in roundworm cells. These proteins instruct the cells to change sugar into energy when they have low cellular energy.
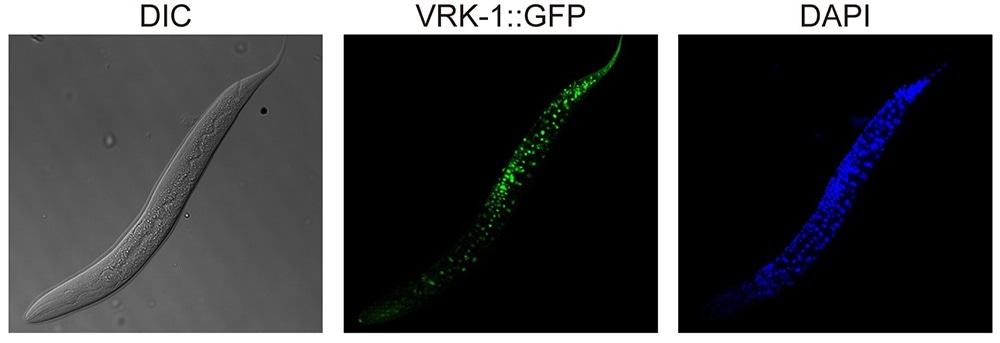
VRK-1 that was visualized by tagging with green fluorescence protein (GFP) in C. elegans. Image Credit: Seung-Jae V. LEE, The Korea Advanced Institute of Science and Technology.
Such proteins are also found in humans, providing fascinating possibilities for creating drugs that promote longevity. These latest discoveries were published in the Science Advances journal on July 1st, 2020.
The roundworm called Caenorhabditis elegans (C. elegans) is a nematode that measures 1 mm and is often used in laboratory testing. This creature enjoyed an increased lifespan when scientists fiddled with a pair of proteins that track the consumption of energy by its cells.
In roundworm cells, the AMPK and VRK-1 proteins work together, with the latter telling the former to get to work by attaching a phosphate molecule, made up of four oxygen and one phosphorus atoms, on it.
In turn, the function of the AMPK protein is to track the cellular energy levels, especially when the energy in the cells is running low. Essentially, the VRK-1 protein controls the AMPK protein, which, in turn, controls the energy status of the cells.
The research team, headed by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST, used a series of different biological research tools, such as introducing foreign genes into the worm, and successfully dialed up and down the genetic activity that tells cells to synthesize the VRK-1 protein. Such a gene has remained largely unchanged all through evolution. A majority of the complex organisms, including humans, have this same kind of gene.
Sangsoon Park, the study’s lead author, and his collaborators proved that the increased production, or overexpression, of the VRK-1 protein increased the lifespan of C. elegans, which usually survives for only two to three weeks, and the suppression of VRK-1 synthesis decreased its lifespan.
The researchers observed that reduced mitochondrial respiration increased the activity of the VRK-1-to-AMPK cellular-energy tracking procedure in low cellular energy status. The reduced mitochondrial respiration denotes the set of metabolic chemical reactions that constitute the oxygen inhaled by the worm to change macronutrients from food into the energy “currency” that is spent by cells to do everything they have to do.
Mitochondria—the energy-producing engine rooms in cells—are already known to play a vital role in aging, and reductions in mitochondrial functions are linked to age-related disorders. On the other hand, the mild suppression of mitochondrial respiration has been demonstrated to support longevity in a wide range of species, like mammals and flies.
When the team performed analogous tinkering with cultured human cells, they observed that they could also simulate the ramp up and down of the VRK-1-to-AMPK procedure that takes place in roundworms.
This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1.”
Seung-Jae V. Lee, Professor, Department of Biological Sciences, The Korea Advanced Institute of Science and Technology
The study, at the very least, has pointed out a fascinating direction for studying novel therapeutic approaches to fight metabolic diseases by targeting the modulation of the VRK-1 protein. In metabolic diseases, including mitochondrial diseases, chemical reactions in the body are disrupted.
However, before longevity drugs or metabolic disorder therapeutics can be considered by researchers, more studies should be performed to further understand how the VRK-1 protein functions to stimulate the AMPK protein, and also understand the accurate mechanics of how the latter regulates cellular energy.
Source:
Journal reference:
Park, S., et al. (2020) VRK-1 extends life span by activation of AMPK via phosphorylation. Science Advances. doi.org/10.1126/sciadv.aaw7824.