Studies on cellular dynamics are vital to interpret the development of cells and the progression of various diseases.
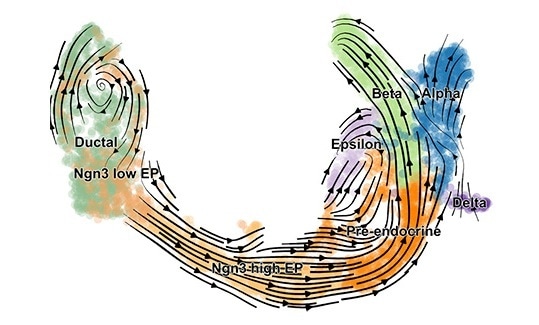
scVelo reveals fine-grained insights into the developmental processes in the pancreas. Image Credit: © Helmholtz Zentrum München.
At Helmholtz Zentrum München and Technical University of Munich (TUM), researchers have developed a machine learning technique and open source software called single-cell velocity (scVelo) to predict the dynamics of gene activity in single cells. This enables biologists to strongly estimate the future state of each cell
Conventional single-cell sequencing techniques help to gain a better understanding of the differences and functions of cells; however, they do this only with static images and not with time-lapse films. Such a restriction renders it difficult to reach conclusions about the dynamics of gene activity and cell development.
The aim of the newly developed technique “RNA velocity” is to rebuild the developmental path of a cell on a computational basis (exploiting ratios of both spliced and unspliced transcripts).
But this technique is relevant only to steady-state populations. Hence, scientists were searching for ways to widen the idea of RNA velocity to dynamic populations that are extremely important to interpret the development of cells and their responses to diseases.
Scientists from the Institute of Computational Biology at Helmholtz Zentrum München and the Department of Mathematics at TUM created single-cell velocity (“scVelo”). This technique predicts the velocity of RNAs with an artificial intelligence (AI)-based model by resolving the whole gene-wise transcriptional dynamics.
This enables the researchers to generalize the idea of RNA velocity to a broad range of biological systems, such as dynamic populations.
We have used scVelo to reveal cell development in the endocrine pancreas, in the hippocampus, and to study dynamic processes in lung regeneration—and this is just the beginning.”
Volker Bergen, Study First Corresponding Author, Helmholtz Zentrum München
Bergen is the main developer of the scVelo method.
Using the scVelo method, scientists can predict the reaction speeds of the transcription, splicing, and degradation of RNAs without requiring any experimental data. Such reaction rates can aid scientists to further interpret the phenotypic heterogeneity and cell identity.
Moreover, a latent time introduced by the researchers rebuilds the unfamiliar developmental time to place the cells along the path of the fundamental biological process. That is specifically handy to gain a deeper understanding of cellular decision making.
Furthermore, the scVelo method demonstrated putative driver genes and regulatory changes therein. With this method, scientists can learn how and why cells are developing the way they do.
Empowering personalized treatments
scVelo is an AI-based tool that leads to personalized therapies. Going from static pictures to complete dynamics enables scientists to shift from descriptive models to predictive models. In the days to come, this may help scientists to figure out the progression of various diseases, like the formation of tumors, or to reveal cell signaling in reaction to cancer treatment.
“scVelo has been downloaded almost 60.000 times since its release last year. It has become a stepping-stone tool towards the kinetic foundation for single-cell transcriptomics.”
Fabian Theis, Professor and Director, Institute for Computational Biology, Helmholtz Zentrum München
Theis, who conceived the research, is also the Chair for Mathematical Modeling of Biological Systems at the Technical University of Munich.
Source:
Journal reference:
Bergen, V., et al. (2020) Generalizing RNA velocity to transient cell states through dynamical modeling. Nature Biotechnology. doi.org/10.1038/s41587-020-0591-3.