According to researchers from the University of Cambridge, G-quadruplexes—that is, four-stranded DNA structures—have been demonstrated to play a crucial role in specific types of breast cancer for the first time. This finding offers a promising new target for personalized medicine.
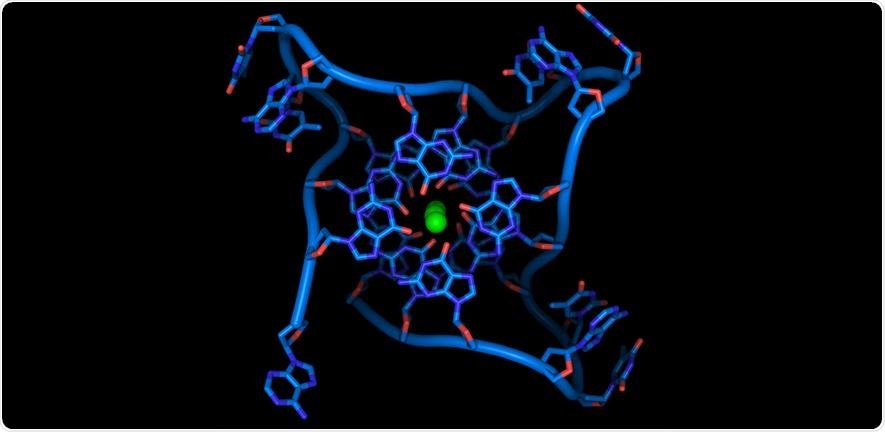
Crystal structure of parallel quadruplexes from human telomeric DNA. The DNA strand (blue) circles the bases that stack together in the center around three co-ordinated metal ions (green). Image Credit: Thomas Splettstoesser.
Earlier in 1953, Francis Crick and James Watson, both scientists from the University of Cambridge, co-authored a study published in the Nature journal. The study demonstrated that DNA found in the human cells has an intertwined “double helix” structure.
Sixty years later, a research team headed by Professor Sir Shankar Balasubramanian and Professor Steve Jackson, also from the University of Cambridge, discovered that a strange four-stranded configuration of DNA can occur across the human genome found in living cells.
Such structures develop in the DNA regions that are rich in guanine (G)—one of its building blocks)—when a single strand of the double-stranded DNA initially loops out and then doubles back on itself, creating a four-stranded “handle” in the genome. Consequently, such structures are known as G-quadruplexes.
Professor Balasubramanian and collaborators have already developed sequencing methods and technologies that can detect G-quadruplexes in DNA as well as in chromatin—a substance containing proteins and DNA.
The researchers have already demonstrated that G-quadruplexes play a significant role in transcription, a major step in reading the genetic code as well as producing proteins from DNA. Significantly, their study also demonstrated that G-quadruplexes could probably occur in cellular genes that are quickly dividing, for example, cancer cells.
For the first time, the researchers have identified where G-quadruplexes develop in the preserved tumor biopsies or tissues of breast cancer. The study results were recently published in the Nature Genetics journal.
The research team, from the University of Cambridge and headed by Professor Balasubramanian and Professor Caldas, applied their quantitative sequencing technique to analyze the structures of the G-quadruplex DNA in 22 tumor models.
The researchers produced these models by initially taking biopsies from patients admitted at Addenbrooke’s Hospital, Cambridge University Hospital NHS Foundation Trust, and subsequently transplanting and growing the tumors in mice.
At the time of cell division and DNA replication that occur during cancer, vast regions of the genome can be incorrectly duplicated many times, resulting in the supposed copy number aberrations (CNAs).
The team discovered that G-quadruplexes are prevalent inside these CNAs, especially inside genes as well as genetic regions that have an active role to play in transcription and hence in fueling the growth of tumor.
We’re all familiar with the idea of DNA’s two-stranded, double helix structure, but over the past decade it’s become increasingly clear that DNA can also exist in four-stranded structures and that these play an important role in human biology. They are found in particularly high levels in cells that are rapidly dividing, such as cancer cells. This study is the first time that we’ve found them in breast cancer cells.”
Sir Shankar Balasubramanian, Professor, University of Cambridge
According to Robert Hänsel-Hertsch, “The abundance and location of G-quadruplexes in these biopsies gives us a clue to their importance in cancer biology and to the heterogeneity of these breast cancers.”
Importantly, it highlights another potential weak spot that we might use against the breast tumour to develop better treatments for our patients.”
Robert Hänsel-Hertsch, Study First Author, Center for Molecular Medicine Cologne, University of Cologne
At least 11 subtypes of breast cancer are believed to exist, and the researchers discovered that each subtype has a different pattern or “landscape” of G-quadruplexes that is exclusive to the transcriptional programs fueling that specific subtype.
While we often think of breast cancer as one disease, there are actually at least 11 known subtypes, each of which may respond in different ways to different drugs. Identifying a tumour’s particular pattern of G-quadruplexes could help us pinpoint a woman’s breast cancer subtype, enabling us to offer her a more personalised, targeted treatment.”
Carlos Caldas, Professor, Cancer Research UK Cambridge Institute, University of Cambridge
If synthetic molecules are used to target the G-quadruplexes, cells could be prevented from replicating their DNA and cell division can thus be inhibited, stopping the proliferation of runaway cells at the root of cancer.
The researchers identified a couple of such molecules—one called pyridostatin and a second compound known as CX-5461, which has earlier been tested in a phase I clinical trial against BRCA2-deficient breast cancer.
Source:
Journal reference:
Hänsel-Hertsch, R., et al. (2020) Landscape of G-quadruplex DNA structural regions in breast cancer. Nature Genetics. doi.org/10.1038/s41588-020-0672-8.