The human skeleton helps in moving the body. Similarly, fine skeleton-like filaments inside the cells of an individual also help in the movement of cellular structures.
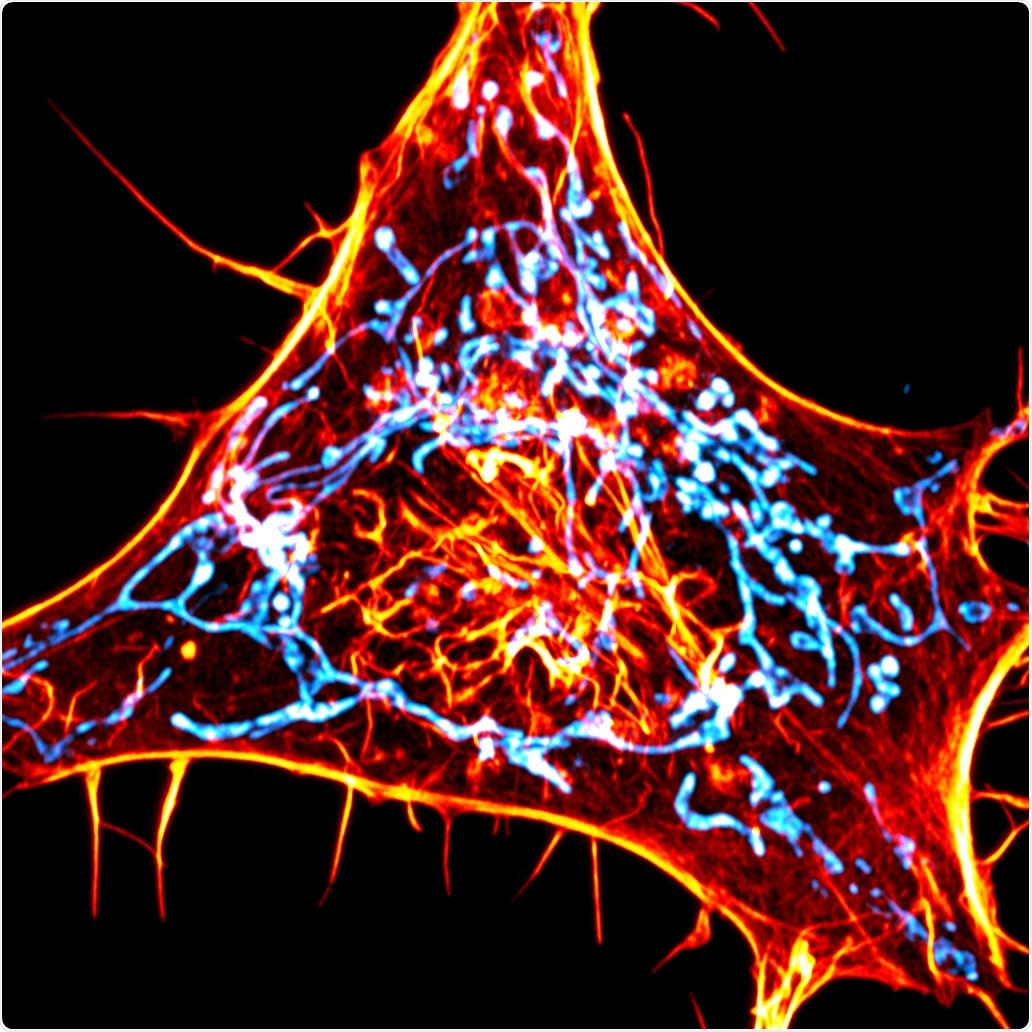
A cancer cell labeled for actin (red) and mitochondria (cyan). The scientists designed novel probes that specifically monitor interactions between actin and mitochondria. Image Credit: Salk Institute/Waitt Advanced Biophotonics Center.
Scientists from the Salk Institute have now created a novel imaging technique that allows them to track actin, which is a small subset of these filaments.
Actin is the most abundant protein in the cell, so when you image it, it’s all over the cell. Until now, it’s been really hard to tell where individual actin molecules of interest are, because it’s difficult to separate the relevant signal from all the background.”
Uri Manor, Study Corresponding Author and Director, Biophotonics Core Facility, Salk Institute
Using the novel imaging technique, the researchers from the Salk Institute have successfully tracked how a crucial function is mediated by actin—that is, helping the “power stations” of the cells called mitochondria to divide into two parts.
Published in the Nature Methods journal on August 10th, 2020, the study could offer a deeper insight into mitochondrial dysfunction that has been associated with neurodegenerative diseases, cancer, and aging.
Mitochondrial fission denotes the process through which such energy-generating structures, or organelles, split and grow in numbers as part of standard cellular maintenance; the organelles divide when a cell itself is dividing and they also divide when cells are under tremendous amounts of stress or when mitochondria are impaired.
But the exact way through which a single mitochondrion divides into a pair of mitochondria has not been clearly understood, specifically how the initial constriction occurs. Studies have discovered that there is minimal mitochondrial fission when actin is fully removed from a cell, among several other effects. This indicates a role for actin in the process.
But according to the team, destroying all the actin leads to extensive cellular defects that makes it difficult to study the exact role of the protein in any one process.
Therefore, Manor and his collaborators created a novel method to image actin. Instead of tagging all the actin found in the cell with fluorescence, the team developed an actin probe to target the external membrane of the mitochondria. When the actin is within 10 nm of the mitochondria, it binds to the sensor leading to increased fluorescence signal.
Instead of seeing the haphazard distribution of actin around all the membranes of the mitochondria, as would be the case if there were no discrete communications between the organelles and actin, Manor’s research team observed vivid hotspots of actin.
On closer observation, the hotspots were situated at the same sites where endoplasmic reticulum—another organelle—crosses the mitochondria, which were earlier found to be the sites of fission.
As the researchers watched the illumination and disappearance of actin over time, they found that 97% of the sites of mitochondrial fission had actin fluorescing around them. The team speculated that the other 3% of fission sites also contained actin but that it was invisible.
This is the clearest evidence I’ve ever seen that actin is accumulating at fission sites. It’s much easier to see than when you use any other actin marker.”
Cara Schiavon, Study Co-First Author and Joint Postdoctoral Fellow, Salk Institute
Schiavon works in the laboratories of Uri Manor and Salk Professor Gerald Shadel.
The team then modified the actin probe so that it is fixed to the membrane of endoplasmic reticulum instead of the mitochondria and successfully pieced together the order in which different kinds of components join the process of mitochondrial fission.
The study results imply that the actin binds to the mitochondria before reaching the endoplasmic reticulum. This offers a valuable understanding of how the mitochondria and endoplasmic reticulum jointly work to coordinate the process of mitochondrial fission.
In further experiments explained in a pre-print manuscript available on bioRxiv, Manor’s research team also reported that the same buildup of endoplasmic reticulum-associated actin is observed at the locations where other cellular organelles, such as peroxisomes, lysosomes, and endosomes, divide.
This implies a wide new role for a subset of actin involved in the homeostasis and dynamics of organelles (physiological equilibrium).
In the days to come, the researchers hope to study genetic mutations and how they change the dynamics of mitochondria and potentially affect interactions between actin and the mitochondria. The team has also planned to adapt the actin probes to view actin that is proximal to other cellular membranes.
This is a universal tool that can now be used for many different applications. By switching out the targeting sequence or the nanobody, you can address other fundamental questions in cell biology.”
Tong Zhang, Study Co-First Author and Light Microscopy Specialist, Salk Institute
“We’re in a golden age of microscopy, where new instruments with ever higher resolution are always being invented; but in spite of that there are still major limitations to what you can see. I think combining these powerful microscopes with new methods that select for exactly what you want to see is the next generation of imaging,” Manor concluded.
Source:
Journal reference:
Schiavon, C. R., et al. (2020) Actin chromobody imaging reveals sub-organellar actin dynamics. Nature Methods. doi.org/10.1038/s41592-020-0926-5.