A team of researchers, headed by Daniel Lietha from The Margarita Salas Center for Biological Research (the Centro de Investigaciones Biológicas Margarita Salas, CIB-CSIC), has recently described the mechanistic details regarding the activation of the Focal Adhesion Kinase (FAK) on lipid membranes. The study was published in The EMBO Journal.
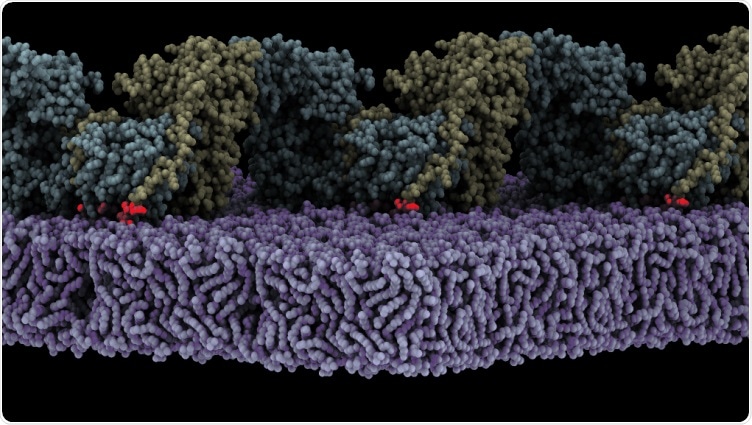
Assembly of oligomeric FAK (yellow/cyan) on the membrane (purple) triggers autophosphosphorylation. Shown is a state where the autophosphorylation site (red glow) is bound to the active site of the FAK kinase. Image Credit: The Margarita Salas Center for Biological Research (CIB-CSIC).
Lietha initiated this study while working at the Spanish National Cancer Research Centre (CNIO) and has now concluded it in his present institution, that is, The Margarita Salas Center for Biological Research.
As a crucial protein, FAK ensures controlled adhesion, proliferation, migration, and survival of cells, which in cancer is usually responsible for causing abnormal invasion of cells and thus results in metastatic cancers.
FAK assumes an autoinhibited state in the cytosol but is stimulated upon recruitment into focal adhesions; however, researchers do not know how this process takes place or what exactly induces structural modifications.
Lietha’s team has shown that FAK is stimulated when it is localized to the cell membrane where it communicates with certain phosphoinositide lipids. Now, using Cryo-Electron Microscopy, the team has acquired a high-resolution structure of an oligomeric form of FAK attached to a lipid membrane.
The structural analysis demonstrated that when FAK initially binds to the membrane, it leads to steric clashes that, in turn, discharge the kinase domain from autoinhibition, enabling it to go through a massive conformational modification and communicate with the membrane in an orientation that positions the active location towards the membrane.
Such a structure also demonstrates that many interfaces align in the reorganized conformation to facilitate the oligomerization of the FAK protein on the membrane, thus exposing a crucial site of phosphorylation. This leads to autophosphorylation and thus stimulation of the FAK protein.
The team simulated molecular dynamics to have a better understanding of the dynamics and mechanism of the autophosphorylation process and further activation on the membrane.
To verify the computational model, the researchers generated different mutants of FAK that carry mutations at the observed interfaces. The team also performed elaborate biochemical experiments to assess how the different kinds of mutations affect the binding of lipids and also the activation and autophosphorylation of the FAK protein.
The team also studied the effect of the mutations on the function of the FAK protein in cancer cells and demonstrated that the uncovered mechanism is crucial for the invasion and proliferation of cancer cells.
Source:
Journal reference:
Acebrón, I., et al. (2020) Structural basis of Focal Adhesion Kinase activation on lipid membranes. The EMBO Journal. doi.org/10.15252/embj.2020104743