Stem cells in the intestines maintain an excellent balance between two promising forms—developing into intestinal epithelial cells, or remaining as stem cells.
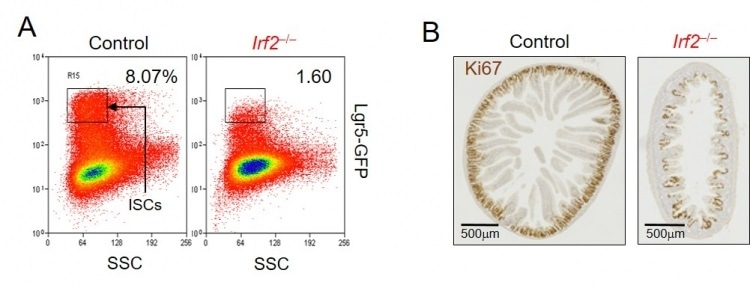
An IRF2 deficiency reduces intestinal stem cells (ISCs) and attenuates crypt regeneration after injury. A. Flow cytometric analysis revealed that the number of ISCs was reduced in Irf2–/– mice compared with control mice. Lgr5-GFP: reporter fluorescence of ISCs. B. Sections of the jejunum from control (left) and Irf2–/– (right) mice, 6 days after the induction of epithelial injury by 5-fluorouracil administration, were stained with Ki67. The number of Ki67-stained regenerated crypts was substantially reduced in Irf2–/– mice compared with control mice. Image Credit: Tokyo Medical and Dental University.
In a new research work, scientists from the Tokyo Medical and Dental University (TMDU) have described a new molecular mechanism that controls this fine balance and maintains the stemness of the intestinal stem cells—in other words, their potential to develop into any type of intestinal epithelial cells.
Intestinal epithelium, the inner lining of intestines, makes sure that nutrients are adequately digested and adsorbed. This intestinal lining is composed of many different types of cell, which collectively meet a particular function.
Stem cells in the intestines ensure appropriate functioning of the intestines, in which damaged and old cells are continuously replaced with young cells by differentiating or developing into one of the different types of intestinal epithelial cells when required.
Since there is a continuous demand for new cells, stem cells in the intestines are capable of renewing on their own, thus offering also a continuous supply of stem cells. But not much is known about the mechanisms that control this balance between differentiation and self-renewal.
Just like any other type of stem cell, intestinal stem cells have the ability to differentiate into any cell within their lineage. But they have to do it in a regulated manner, only differentiating when needed. The goal of our study was to understand the regulatory mechanism that preserves the stemness of intestinal stem cells.”
Toshiaki Ohteki, Study Corresponding Author and Professor, Tokyo Medical and Dental University
To realize their objective, Taku Sato, who significantly contributed to this study, and colleagues targeted a molecular signaling pathway that they had earlier demonstrated to maintain the stemness of hematopoietic stem cells (HSCs) that lead to the development of blood cells.
Interferons are molecules that are particularly synthesized during bacterial and viral infections; however, more recently, it was also demonstrated that these molecules exist even where there are no infections to control many biological processes.
In both cases, interferons promote specific genes to be expressed—a procedure controlled by the protein interferon regulatory factor-2 (IRF2) to ensure that the activities of interferons are balanced. With regard to HSCs, IRF2 was found to be a crucial factor for their stemness.
In the latest study, the team noted that IRF2 is synthesized all through the intestinal epithelium and that mice deficient with IRF2 had normal anatomical structure at the time of homeostasis—the absence of an infection or any other impairing factor.
But in the presence of 5-fluorouracil, which impairs the intestinal epithelium, the team found that normal mice were able to regenerate fully; however, mice that lacked the IRF2 exhibited a blunted regenerative response, denoting that stem cells in the intestines were unable to work efficiently in the absence of IRF2.
Fascinatingly, immature Paneth cells, which are essentially specialized secretory cells, were extremely abundant in mice that lacked the IRF2. The team observed the same result in normal mice subjected to lymphocytic choriomeningitis virus (LCMV), which is responsible for causing chronic infections.
These are striking results that show how excess interferon signaling in the absence of IRF2 impairs the ability to self-renew and directs intestinal stem cells towards the secretory cell lineage. Our findings provide new insight into the biology of intestinal stem cells and show that regulated interferon signaling is a means to preserve the stemness of intestinal stem cells.”
Toshiaki Ohteki, Study Corresponding Author and Professor, Tokyo Medical and Dental University
Source:
Journal reference:
Sato, T., et al. (2020). Regulated IFN signalling preserves the stemness of intestinal stem cells by restricting differentiation into secretory-cell lineages. Nature Cell Biology. doi.org/10.1038/s41556-020-0545-5.