Researchers had identified an actin (cellular protein) cytoskeketal nucleator, called Arp2/3 complex, more than 20 years ago. This complex plays a major role in neurodevelopment, immune response, cell division, and other biological processes.
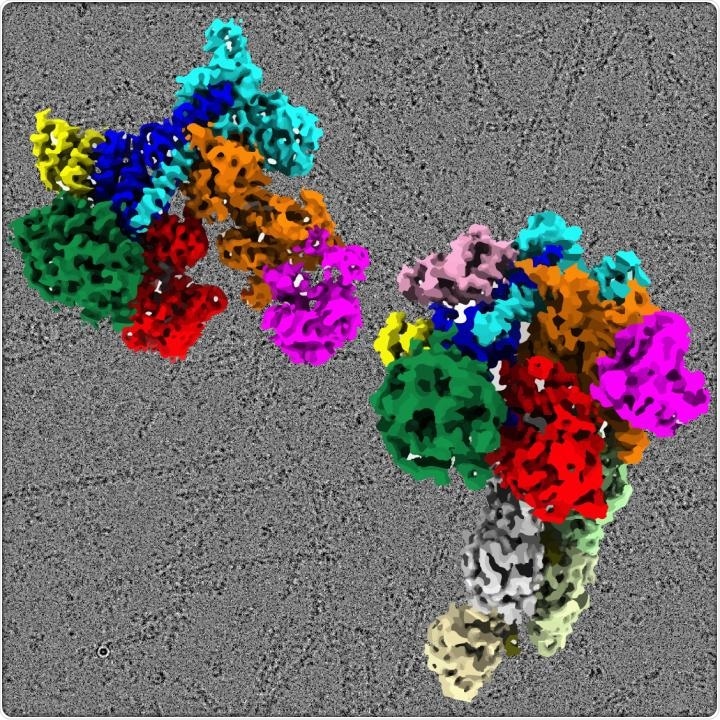
An artistic representation of the Arp2/3 complex structure in an inactive state (top left) and active state with nucleated filament (bottom right). With a cryo-EM micrograph as the background, it shows actin filaments nucleated from the Arp2/3 complex. Image Credit: Stony Brook University.
However, no established structure of the activated form of the Arp2/3 complex was available until now, representing a major breakthrough that may lay the groundwork for unraveling its function in the field of biology and also in disease development.
Stony Brook University researchers, headed by Saikat Chowdhury, PhD, have now successfully determined the structure of the Arp2/3 complex and described it in an article published in the Nature Structural & Molecular Biology journal.
Chowdhury, and the study’s first author and graduate student Mohammed Shaaban, used the cryo-electron microscopy technique and eventually established the first near-atomic resolution structure of the activated state of the Arp2/3 complex.
The structure reveals the activated state of the Arp2/3 complex attached to a signaling molecule. It even reveals the nucleated actin filament, thereby offering a structural picture of the local and global conformational variations in the Arp2/3 complex through which a new actin filament is able to grow in cells.
Obtaining the macromolecular structure of activated Arp2/3 complex has been a long-standing goal for scientists. Our structure reveals a level of molecular details which show the individual components of the complex and how they are positioned relative to each other in the active state.”
Saikat Chowdhury, PhD, Study Senior Author and Assistant Professor, Department of Biochemistry and Cell Biology, College of Arts and Sciences, Stony Brook University
A structure of the activated state of the Arp2/3 complex will allow scientists to perform more comprehensive studies on this complex. Chowdhury elaborated that this is very significant because when the Arp2/3 complex is deregulated in the biological form, it could lead to neurodegeneration, viral and bacterial infections, cancer metastasis, and wound healing problems.
So not only does this structure enable us to fill a knowledge gap in the actin cell biology field, it potentially helps to build our understanding of the underlying causes of a number of diseases with the ultimate goal of developing new therapeutics.”
Saikat Chowdhury, PhD, Study Senior Author and Assistant Professor, Department of Biochemistry and Cell Biology, College of Arts and Sciences, Stony Brook University
To establish the Arp2/3 structure in its activated form, the team had to use the high-performance computing capabilities in the Division of Information Technology and technologies available in the Cryo-Electron Microscopy Facility at Stony Brook University; the facility is a center assisted by the National Institutes of Health (NIH)
Chowdhury is also affiliated with the Institute of Engineering Driven Medicine and Institute of Chemical Biology & Drug Discovery at Stony Brook University, and is also an affiliated scientist at Brookhaven National Laboratory.
The study was performed in association with Brad Nolen from the University of Oregon, and partly funded by the NIH and SUNY, Stony Brook University.
Source:
Journal reference:
Shaaban, M., et al. (2020) Cryo-EM reveals the transition of Arp2/3 complex from inactive to nucleation-competent state. Nature Structural & Molecular Biology. doi.org/10.1038/s41594-020-0481-x.