Obesity, the leading cause of type 2 diabetes and associated chronic illnesses, will collectively kill more people around the world in 2020 than the current Covid-19 coronavirus.
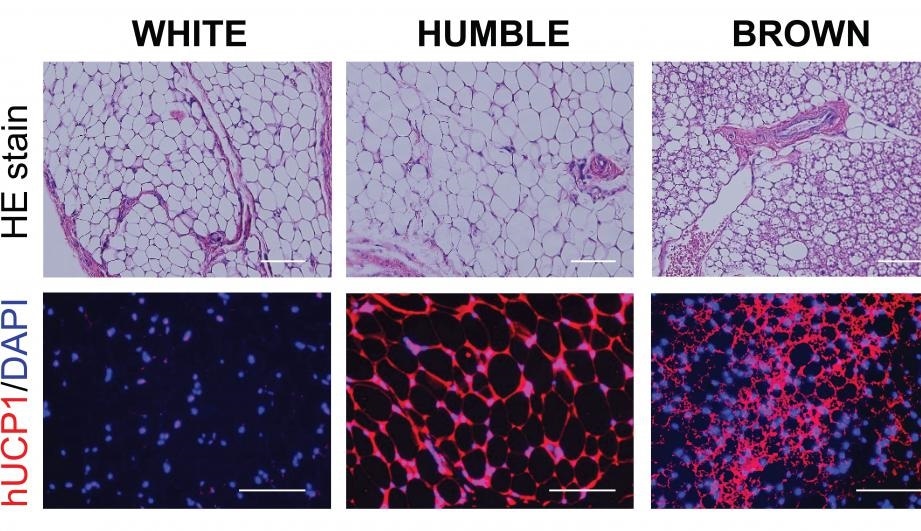
Microscopic images of the various types of fat tissues developed in mice after transplantation. Top panels show the fat tissue’s general morphology, and the bottom panels are the tissue sections stained with hUCP1 (red color), which is unique for brown fat cells. These images show that while the HUMBLE fat cells are morphologically similar to the white fat cells, they express the brown fat specific hUCP1 protein. Image Credit: Joslin Diabetes Center.
Now, researchers from Joslin Diabetes Center have provided a proof of concept for a new cell-based treatment against this dangerous medical condition.
According to Yu-Hua Tseng, PhD, a Senior Investigator in the Section on Integrative Physiology and Metabolism at Joslin Diabetes Center, the promising treatment for obesity involves transplanting human brown-like (HUMBLE) fat cells—human white fat cells that have been genetically manipulated to become analogous to heat-generating brown fat cells.
Tseng, who is also the study’s senior author of the article published in the Science Translational Medicine journal, added that unlike the white fat cells, the brown fat cells burn energy rather than storing energy. During the process, brown fat can reduce extreme glucose and lipid levels in the blood that are associated with metabolic disorders, like diabetes.
But individuals, who are obese or overweight, have less of this beneficial brown fat—an obstacle that HUMBLE cells are made to overcome, added Tseng.
Along with her collaborators, Tseng produced the cells from human white fat cells in a progenitor phase—which are yet to be fully developed into their final fat form. The team employed a variant of the CRISPR-Cas9 genome editing system to increase the expression of a gene known as UCP1. This gene activates white fat cell progenitors to form into brown fat-like cells.
Tseng, who is also a professor of medicine at Harvard Medical School, further added that when the HUMBLE progenitor cells were transplanted into the immune system-deficient mice, they developed into cells that worked almost similar to the own brown fat cells of mice.
Tseng’s team then compared transplants of these cells against the original white fat cells in mice that were given a high-fat diet. Mice that received the HUMBLE transplants exhibited relatively greater sensitivity to insulin and had the potential to remove glucose from the blood—two major factors that are damaged in type 2 diabetes.
In addition, the mice that received HUMBLE transplants put on less weight when compared to the mice that received the transplanted white fat cells, persisting in the same range as animals that received brown fat cells.
Perhaps unexpectedly, the Joslin team showed that these advantages were largely due to signals produced from the transplanted cells to endogenous, or prevalent, brown fat cells found in the mice.
Cells in different tissues communicate with each other. In this case, we found that our transplanted HUMBLE cells secrete a molecule called nitric oxide, which is carried by red blood cells to the endogenous brown cells and activates those cells.”
Yu-Hua Tseng, PhD, Senior Investigator, Section on Integrative Physiology and Metabolism, Joslin Diabetes Center
According to Tseng, if the HUMBLE method continues to work in pre-clinical research, it might ultimately be possible to produce this cell type for individual patients. A procedure like this would eliminate a small amount of the white fat cells in patients, separate the progenitor cells, alters those cells to increase the expression of UCP1, and subsequently return the resulting HUMBLE cells to the patient.
But that individualized method would be costly and complex; therefore, the Tseng laboratory is looking for two alternative methods that may prove to be more viable for clinical use.
One option is to employ cells that are not personalized but rather encapsulated through biomaterials that safeguard the cells against rejection by the immune system of a patient. (The Joslin team and their colleagues have long analyzed such materials for cell transplants for type 1 diabetes.)
The other alternative is gene therapies in which the UCP1 gene is directly expressed in white fat progenitor cells in the body, so that such cells gain the HUMBLE-like traits.
Tseng emphasized that the new study is progressing in spite of the Covid-19 pandemic, which puts diabetic people at a higher risk of developing adverse outcomes if they get infected.
Employing cell-based or gene therapies to treat obesity or type 2 diabetes used to be science fiction. Now scientific advances, such as CRISPR gene-editing technologies, will help us to improve the metabolism, the body weight, the quality of life and the overall health of people with obesity and diabetes.”
Yu-Hua Tseng, PhD, Senior Investigator, Section on Integrative Physiology and Metabolism, Joslin Diabetes Center
Joslin research: HUMBLE cells can improve metabolic health
Scientists at Joslin Diabetes Center have delivered a proof of concept for a novel cell-based therapy against this dangerous condition. Video Credit: Joslin Diabetes Center.
Source:
Journal reference:
Wang, C.-H., et al. (2020) CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Science Translational Medicine. doi.org/10.1126/scitranslmed.aaz8664.