Gastric cancer is one of the major causes of cancer-related mortality around the world. It is known for its potential to spread across the peritoneal cavity.
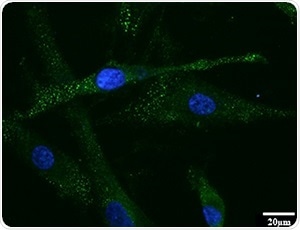
Expression of FAP was found in HPMCs treated with IL-17A using immunofluorescence staining. This means HPMCs were transformed into myofibroblast, so-called CAF, by IL-17A. FAP: fibroblast activation protein as a marker of CAF. HPMCs: human peritoneal mesothelial cells. CAF: cancer associated fibroblast. Image Credit: Kanazawa University.
Metastatic gastric cancer cells, in addition to causing secondary tumors in other organs, activate widespread stromal fibrosis, or lead to the development of scar tissue, which can be more lethal than the cancer itself. For example, bowel obstruction, jaundice, and hydronephrosis are all common side effects of gastric cancer-associated fibrosis.
Moreover, the heavily packed scar tissue can prevent chemotherapy drugs from making it to their target because of intra-tumoral high pressure.
Therefore, inhibiting fibrosis could enhance the prognosis for patients suffering from gastric cancer. But the issue is, scientists have still not identified the causes that lead to fibrosis, let alone how to inhibit it.
However, in a study recently published in the Gastric Cancer journal, Kanazawa University, researchers observed that an inflammatory protein created by mast cells, called IL-17A, activates cellular modifications in the peritoneum, resulting in stromal fibrosis in patients affected by gastric cancer.
Katsuya Gunjigake, the study’s lead author from the Division of Cancer Medicine at Kanazawa University, explained why IL-17A was targeted by the team.
Over-stimulation of the immune system by IL-17A plays a major role in chronic inflammatory diseases such as rheumatoid arthritis and multiple sclerosis. It has also been associated with increased tumor growth and dissemination in various forms of cancer. Interestingly though, while studies had shown that IL-17A causes fibrosis in both Crohn’s disease and lung disease, no one had investigated the link between tissue fibrosis and IL-17A in cancer.”
Katsuya Gunjigake, Study Lead Author, Division of Cancer Medicine, Kanazawa University
When the team examined cancerous tissue from a total of 70 gastric cancer patients, who had peritoneal dissemination, they observed that the extent of fibrosis was controlled by the amount of IL-17A, and that a subgroup of white blood cells, known as mast cells, was producing IL-17A.
Mast cells are most commonly associated with anaphylaxis but are also involved in pathogen defense and immune tolerance, among other things. They contain small particles called granules that are filled with molecules such as histamine, serotonin, and IL-17A that are released into the extracellular environment in a process known as degranulation.”
Katsuya Gunjigake, Study Lead Author, Division of Cancer Medicine, Kanazawa University
The team subsequently injected gastric cancer cells and human peritoneal cells into mice and studied the impacts of IL-17A treatment, with fascinating results.
Not only did IL-17A increase tumor size and the degree of fibrosis, it also changed the structure of the peritoneal cells, enhancing their invasive and migratory capabilities. Given the obvious role of IL-17A in driving fibrosis, our results suggest that suppression of mast cell degranulation may be a promising treatment strategy for gastric cancer patients with peritoneal dissemination.”
Sachio Fushida, Study Corresponding Author, Kanazawa University
Source:
Journal reference:
Gunjigake, K., et al. (2020) Interleukin-17A derived from mast cells contributes to fibrosis in gastric cancer with peritoneal dissemination. Gastric Cancer. doi.org/10.1007/s10120-020-01092-2.