Methicillin-resistant Staphylococcus aureus (MRSA) is the single most lethal drug-resistant bacteria in the United States and is also one of the most common bacterial pathogens.
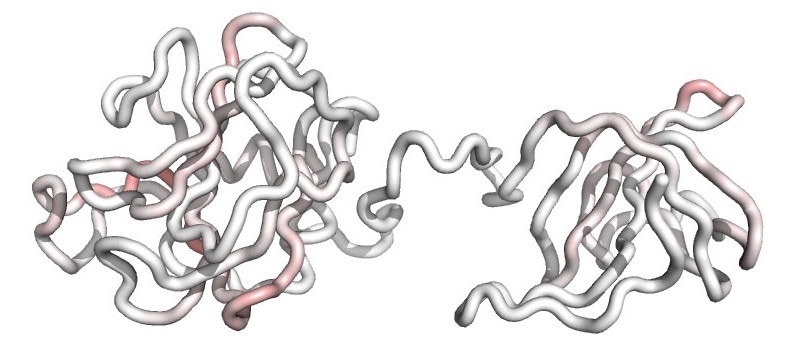
A molecular model of the F12 biotherapy shows the fold of its polypeptide backbone. Residual T-cell epitopes are mapped onto the structure and colored from white (no epitopes) to dark red (dense overlapping epitopes). The muted red coloring indicates that T-cell epitopes have been broadly silenced in the engineered F12 variant, and this reduced epitope content underlies the molecule’s improved safety and efficacy profile. Image Credit: Dartmouth Engineering.
The Centers for Disease Control and Prevention (CDC) has now underscored the importance of identifying an effective treatment against these pathogens.
A new study, headed by Dartmouth Engineering faculty, can help develop a lysin-based antibacterial agent that may allow safe, repeated dosing to treat life-threatening infections caused by various types of S. aureus, including the Methicillin-resistant Staphylococcus aureus.
Lysins are enzymes that are naturally produced by microorganisms and related viruses. In the recent past, these enzymes have demonstrated an ability to treat S. aureus, which can quickly gain resistance to different types of antibiotic medications.
Lysins are one of the most promising next-generation antibiotics. They kill drug-sensitive and drug-resistant bacteria with equal efficacy, they can potentially suppress new resistance phenotypes, and they also have this laser-like precision.”
Karl Griswold, Study Corresponding Author and Associate Professor of Engineering, Thayer School of Engineering at Dartmouth
Although lysins show immense promise, advancement has been slowed down because there were concerns that these enzymes prompt the immune systems of humans to develop antidrug antibodies, which can lead to adverse side effects, such as life-threatening hypersensitivity reactions.
That is the reason why the Dartmouth Engineering group—which also comprised scientists from the computer science department at Dartmouth, The Lundquist Institute at Harbor-UCLA Medical Center, Lyticon, and Stealth Biologics—designed and patented the novel lysin-based antibacterial agent, F12.
In essence, F12 is capable of hiding from the human immune system (because of the deletion of the T cell epitope), and thus does not lead to the same adverse side effects as natural, unmodified lysins.
As the first lysin-based treatment, F12 can be utilized numerous times on a single patient and is thus perfect for treating specifically persistent infections that are sensitive and resistant to drugs.
According to preclinical studies, the efficacy of the F12 antibacterial agent does not reduce with repeated doses, whereas a couple of other anti-MRSA lysin treatments being tested in clinical trials are simply developed to be used only a single time.
We have engineered this super potent, super effective anti-MRSA biotherapeutic, and we’ve done it in a way that renders it compatible with and largely invisible to the human immune system. By making it a safer drug, we've enabled the possibility of dosing multiple times in order to treat even the most highly refractory infections.”
Karl Griswold, Study Corresponding Author and Associate Professor of Engineering, Thayer School of Engineering at Dartmouth
The study titled, “Globally deimmunized lysostaphin evades human immune surveillance and enables highly efficacious repeat dosing,” was recently published in the Science Advances journal. It was the outcome of two grants from the National Institutes of Health (NIH) totaling $1.7 million.
The study also describes the positive outcomes of the treatment in rabbits, mice that have partially humanized immune systems, and research works with extracted human immune cells.
According to Griswold, the antibacterial agent may soon be available for human clinical trials by 2023.
This is the first report of a translation-ready deimmunized lysin, and F12 has serious, bonafide clinical potential.”
Karl Griswold, Study Corresponding Author and Associate Professor of Engineering, Thayer School of Engineering at Dartmouth
Additional studies of the F12 antibacterial agent will look at the synergy with standard-of-care antibacterial chemotherapies; initial outcomes indicate that the combinations are highly strong and inhibit drug-resistance phenotypes.
Source:
Journal reference:
Zhao, H., et al. (2020) Globally deimmunized lysostaphin evades human immune surveillance and enables highly efficacious repeat dosing. Science Advances. doi.org/10.1126/sciadv.abb9011.