For the first time, researchers have detected the structure of a protein fiber associated with early-onset type 2 diabetes.
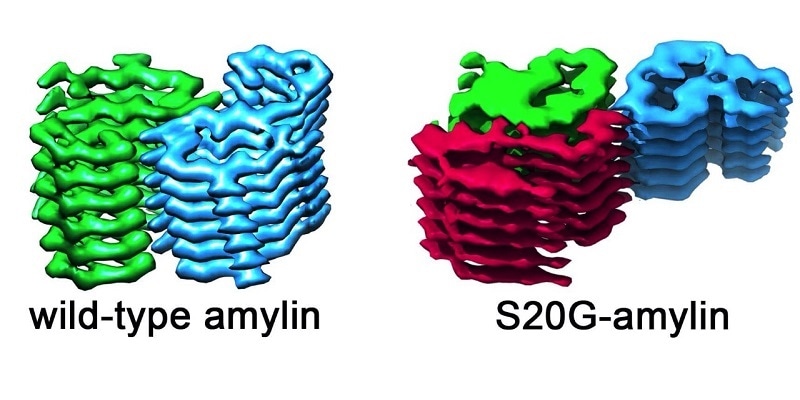
The image shows the structure of the amyloid fibrils in the wild-type amylin and also in the S20G variant. On the left of the image, fibrils from wild-type amylin are made up from layers containing two molecules that are stacked side by side. In the S20G variant, there is a third molecule per layer. This structural arrangement could lead to a greater propensity for aggregation. Image Credit: University of Leeds.
Amylin is a form of protein that controls glucose levels in the body. This small peptide hormone can clump or aggregate together to create amyloid fibrils—a fiber-like structure.
Such aggregates are said to be a hallmark of type 2 diabetes—even though researchers are still unclear how the formation of amyloid causes the disease.
The development of amyloid fibrils is also associated with other diseases, such as neurodegenerative disorders Huntingdon’s, Alzheimer’s, and Parkinson’s disease.
In a study published recently in the Nature Structural &Molecular Biology journal, scientists from the University of Leeds have reported that they have successfully visualized the structure of amylin fibrils using the new electron microscope technology.
The researchers have found an architecture, which according to them, renders certain amylin sequences more prone to develop amylin aggregates than others—a trait associated with earlier onset of type 2 diabetes.
The team compared amylin fibrils collected from the type of amylin found in a majority of the human population— investigators call this wild-type—and compared it with a genetic variant, known as S20G, found in individuals who have early-onset type 2 diabetes.
By examining scores of images, the researchers were able to observe how amylin molecules aggregate into fibrils—creating a complex structure where amylin molecules stack up similar to rungs in a ladder—and to explain the in-depth interactions that hold these fibrils together.
The images demonstrated that the fibrils created by the S20G and wild-type versions of amylin are not the same. All the wild-type fibrils have two amylin copies for each rung.
This was also true for certain S20G fibrils, but most significantly, the team also identified a form of the S20G fibrils that have three amylin fibrils for each layer. This indicates that fibrils can create templates onto which more amylin copies can lock.
This may explain why the S20G-variant protein tends to aggregate more rapidly and is connected to a more rapid onset of type 2 diabetes.
This is a really exciting result because it reveals a mechanism for how ever-larger aggregates might form and that is crucial in understanding the disease process. We know this happens in disease but we have never understood clearly how it happens. Now with these structures we're getting the first glimpse what might be going on.”
Neil Ranson, Study Co-Lead and Professor of Structural Molecular Biology, University of Leeds
Ranson is also the Deputy Director of the Astbury Centre for Structural Molecular Biology at the University of Leeds.
According to Sheena Radford FRS, Astbury Professor of Biophysics, Director of the Astbury Centre, and also the study’s co-lead, “We know that the S20G protein aggregates more quickly, and this study provides the rationale as to why that might be the case. This is important, not just for understanding amylin - but for understanding many amyloid diseases in which run-away fibril formation occurs.”
The team applied the method of cryo-electron microscopy to demonstrate the structure of the fibrils. The protein sample was frozen and subsequently examined in an electron microscope to a resolution where individuals can observe individual atoms.
The electron microscopes are accommodated within the Astbury Biostructure Laboratory—a major international research center for structural biology founded in 2016 with financial support from the University of Leeds and the Wellcome Trust.
A better understanding of amyloid structures, like those presented in this study, may lead to a more improved diagnosis and treatment of amyloid disorders through customized treatments based on the type of fibrils formed.
This is just the beginning of the journey to find new ways of combating amyloid disease and was impossible before the new powerful methods of electron microscopy were developed.”
Sheena Radford FRS, Study Co-Lead and Astbury Professor of Biophysics, University of Leeds
Source:
Journal reference:
Gallardo, R., et al. (2020) Fibril structures of diabetes-related amylin variants reveal a basis for surface-templated assembly. Nature Structural & Molecular Biology. doi.org/10.1038/s41594-020-0496-3.