A team of researchers from the Institute of Molecular Biology of the Austrian Academy of Sciences and the University of British Columbia has discovered how a specific protein called JAGN1 plays a crucial role in the production of antibodies and how the body is able to fight against pathogens, such as viruses.
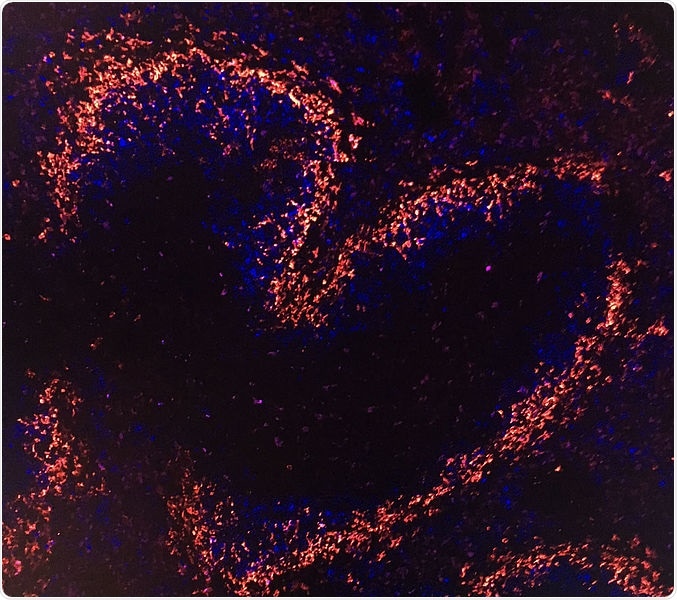
Tile-scan of a mouse spleen stained with MOMA1 (red) and CD23 (blue) and shows splenic architecture as assessed by immunohistochemical staining for the B cell marker CD23, and the metallophilic macrophage marker MOMA1 to visualize splenic marginal zones. Image Credit: © Institute of Molecular Biotechnology/Hagelkruys.
The study results were recently published online in the Journal of Experimental Medicine.
Antibodies play a primary role in medicine, and immune response mediated by antibodies is the ultimate target in the search for a vaccine to overcome the ongoing pandemic. These tiny proteins, also known as immunoglobulins, are utilized by the body as a crucial deterrent against the invading pathogens.
The proteins do this by detecting and tagging foreign substances so that they can be detected by the immune system. These proteins also help to kill the invading pathogens with the aid of other kinds of immune cells.
JAGN1 as a player on the immune system’s team
Dr. Josef Penninger’s team from the Institute of Molecular Biology and from the University of British Columbia, where he is a professor in the Department of Medical Genetics and the director of the Life Sciences Institute, turned their attention on the role of a certain protein known as JAGN1 in the synthesis of antibodies.
The JAGN1 gene was already identified to play a role in the body’s immune system by the Penninger laboratory in association with the Klein laboratory at LMU in Munich among patients suffering from severe congenital neutropenia (SCN)—a disorder that results from a mutation in the JAGN1 gene.
Patients with this disorder have abnormally low concentrations of white blood cells known as neutrophils and suffer from severe infections because their immune systems fail to effectively destroy fungal or bacterial invaders.
In the new study, the team offered a better understanding of the role of JAGN1 with respect to B-cells—white blood cells that can form into plasma cells when they detect foreign substances (antigens), like viruses, bacteria, chemicals, and pollen.
B-cells, in plasma cell form, can create a huge amount of antibodies every second that target a certain antigen. This production takes place at a site inside the cell called the endoplasmic reticulum.
As a finishing touch on their synthesis, the antibodies are “decorated” with sugar molecules on their Fc region—an area that interplays with different parts of the immune system.
Such a process, known as glycosylation, enables the antibody to attach to other immune cells, thus reinforcing the defensive reaction of the body. Typical sugar structures that are bound to proteins are known to influence protein communication and stability between cells and their surroundings.
When we knocked out JAGN1 in B-cells of mice, we were able to measure a drastic reduction in the number of antibodies. In addition, their glycosylation pattern—the addition of specific sugar molecules – was altered on antibodies of JAGN1-deficient B-cells.”
Dr Astrid Hagelkruys, Study First Author and Senior Research Associate, Institute of Molecular Biotechnology
Dr. Hagelkruys continued, “JAGN1 seems to influence the antibody factories in the cells,” says Dr. Penninger. “To our surprise, this change in the sugar structure also leads to a better ability of the antibodies to bind to other immune cells and strengthens the defense reaction.”
The researchers were also able to show this mechanism in human samples.
Rare genetic defects occur in only a handful of people, but they can sometimes help us decipher basic principles of biology. In this case, we were able to prove that a certain gene affects the endoplasmic reticulum and is therefore essential for the mass production of antibodies. At the same time, we also found out that the ‘sugar coat’ of antibodies is changed, which has an important effect on how such antibodies actually work in the body.”
Dr Josef Penninger, Professor, Department of Medical Genetics, Institute of Molecular Biotechnology
Source:
Journal reference:
Hagelkruys, A., et al. (2020) A crucial role for Jagunal homolog 1 in humoral immunity and antibody glycosylation in mice and humans. Journal of Experimental Medicine. doi.org/10.1084/jem.20200559.