Hallucinogenic drugs have been studied for many years, but despite this fact, not much is known about the fundamental mechanisms in the brain through which these drugs induce their hallucinogenic—and (for disorders such as anxiety and depression) potential therapeutic—effects.
This illustration shows the complete cryo-EM structure of a hallucinogen bound to a serotonin receptor. Image Credit: Kim et al. /Cell.
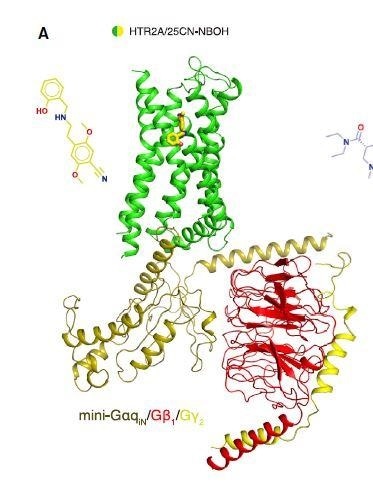
An article, published in the Cell journal on September 17th, 2020, unraveled the first X-ray crystallography structure of LSD attached to its target in the brain—that is, the serotonin receptor. The article also describes the first cryo-electron microscopy (cryo-EM) structure of a prototypical hallucinogen parried with the whole serotonin receptor complex.
Millions of people have taken these drugs recreationally, and now they're emerging as therapeutic agents. Gaining this first glimpse of how they act at the molecular level is really, really important, and it's key to understanding how they work.”
Bryan Roth, Study Senior Author and Professor, Department of Pharmacology, University Of North Carolina SchooloOf Medicine
According to researchers, activation of the 5-HT2A serotonin receptor (HTR2A) in the brain is crucial to the effects of hallucinogenic medications.
These receptors are expressed at very high levels in the human cerebral cortex. When they're activated, they cause neurons to fire in an asynchronous and disorganized fashion, putting noise into the system. We think that's one of the reasons they cause a psychedelic experience. However, it's not at all clear how they exert their therapeutic actions.”
Bryan Roth, Study Senior Author and Professor, Department of Pharmacology, University Of North Carolina School Of Medicine
In the latest study, Roth teamed up with Georgios Skiniotis in this research, a structural biologist at the Stanford University School of Medicine.
According to Skiniotis, “A combination of several different advances allowed us to do this research. One of these is better, more homogeneous preparations of the receptor proteins. Another is the evolution of cryo-EM technologies, which allows us to obtain high-resolution structures of complexes without having to crystalize them.”
The researchers have described the first X-ray crystallography structure of LSD attached to HTR2A in the article.
Moreover, the cryo-EM method was employed to reveal pictures of a prototypical hallucinogen, known as 25-CN-NBOH, attached together with the whole receptor complex, including the effector protein called Gαq. Within the brain, the receptor complex modulates the discharge of neurotransmitters and influences several neurological and biological processes.
Roth is a psychiatrist and biochemist, and heads the Psychoactive Drug Screening Program, financially supported by the National Institute of Mental Health. This gives Roth access to hallucinogenic medications for research purposes. Generally, such compounds cannot be studied easily in the laboratory because they are controlled by the Drug Enforcement Agency as Schedule 1 drugs.
According to Roth, “One of the main goals of my work is to understand how hallucinogens exert their actions on the brain. If we can understand how they work at the molecular level, this will ultimately give us clues into human consciousness, perception, and awareness.”
As a next step, the researchers intend to apply their discoveries in the future to structure-based drug discovery for novel therapeutics. One of the objectives is to identify prospective candidates that may be able to provide therapeutic benefit without any psychedelic impacts.
The more we understand about how these drugs engage and activate the receptors, the better we'll understand their signaling properties. This work doesn't give us the whole picture yet, but it's an important piece of the puzzle.”
Georgios Skiniotis, Structural Biologist, Stanford University School of Medicine
Source:
Journal reference:
Kim, K., et al. (2020) Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell. doi.org/10.1016/j.cell.2020.08.024.