With the growing surge in the COVID-19 cases, scientists are working actively to develop therapies and vaccines to impede SARS-CoV-2—the virus that causes the disease.
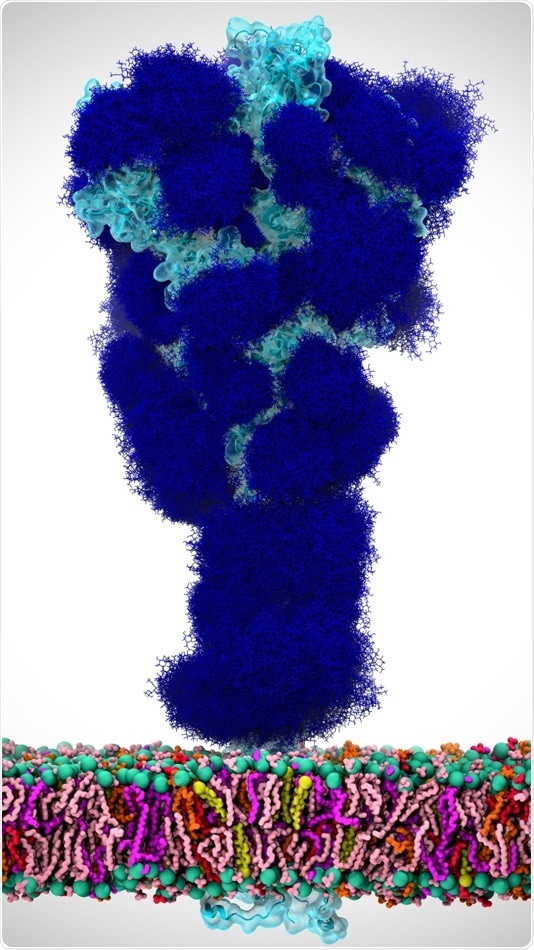
In this illustration, glycans (dark blue) coat the SARS-CoV-2 spike protein (light blue), which is anchored in the viral envelope (colorful bilayer on bottom). Image Credit: Adapted from ACS Central Science 2020.
Several efforts target the coronavirus spike protein, which attaches to the angiotensin-converting enzyme 2 (ACE2) on human cells to allow the entry of the virus.
Scientists have now revealed an active role played by glycans––sugar molecules that can adorn proteins––in this process, indicating targets for vaccines and therapies. The study has been published in the ACS Central Science journal.
Just before the SARS-CoV-2 spike protein can communicate with the ACE2 located on a human cell, it alters its shape to express its receptor binding domain (RBD), the section of the protein that communicates with ACE2. Similar to several viral proteins, the SARS-CoV-2 spike protein has a dense coat of glycans present on its surface.
These glycans, which are bound at particular sites, help protect the viral proteins from the host immune system. Rommie Amaro and collaborators from the University of California San Diego, Maynooth University (Ireland), and the University of Texas at Austin speculated whether some glycans in the SARS-CoV-2 spike protein might also play an active role in the process that leads to infection.
To determine this, the team employed glycomic and structural data to construct molecular dynamics simulations of the SARS-CoV-2 spike protein integrated in the viral membrane. The computational models, which provided a comprehensive picture of each atom in the spike glycoprotein, showed that N-glycans connected to the spike protein at particular sites (N234 and N165) stabilized the shape change that exposes the RBD, which could lead to infection.
The simulations also detected areas of the spike protein that were not coated by glycans and therefore could be susceptible to antibodies, specifically after the shape undergoes modifications.
In lab experiments using biolayer interferometry, the researchers demonstrated that mutating the spike protein so that it no longer had glycans at N234 and N165 decreased the ability to attach to ACE2. These findings lay the groundwork for novel approaches to combat the pandemic, concluded the researchers.
Source:
Journal reference:
Casalino, L., et al. (2020) Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Central Science. doi.org/10.1021/acscentsci.0c01056.