A mice study has revealed a novel gene that is crucial for short-term memory but works in a part of the brain that is not usually linked to memory.
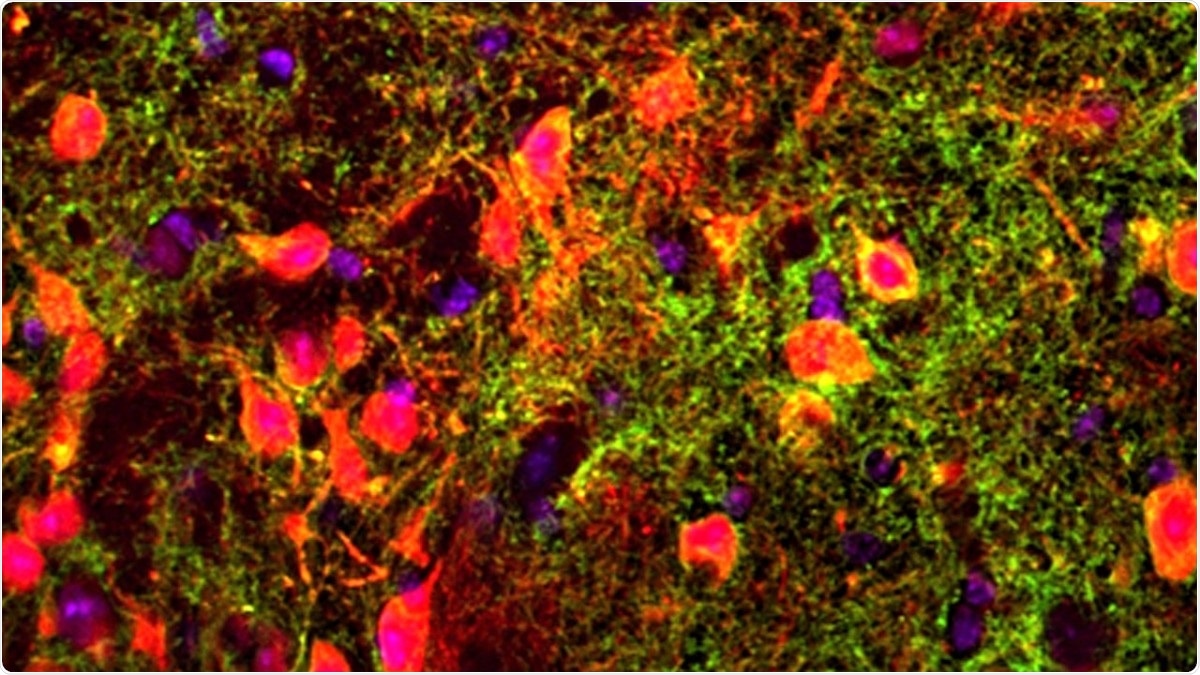
The image shows neurons in the thalamus with GPR12 protein receptors (red), with the receptors themselves (green) lying in terminals at the end of axons that stretch away from each cell’s body. Purple marks other neurons. Image Credit: Kuangfu Hsiao.
The study titled, “A Thalamic Orphan Receptor Drives Variability in Short Term Memory,” was published in the Cell journal on September 29th, 2020.
Traditional studies of short-term memory have focused on the brain’s prefrontal cortex region. But recent studies have indicated that other parts of the brain may also play a role.
Hence, to identify new genes as well as brain circuits that are significant for short-term memory, the scientists investigated genetically diverse mice, instead of inbred mice usually employed in research.
We needed a population that is diverse enough to be able to answer the question of what genetic differences might account for variation in short-term memory,”
Praveen Sethupathy '03, Study Senior Author and Associate Professor of Biomedical Sciences, College of Veterinary Medicine, Cornell University
Sethupathy is also the director of the Cornell Center for Vertebrate Genomics.
Priya Rajasethupathy '04, the Jonathan M. Nelson Family Assistant Professor and head of the Laboratory of Neural Dynamics and Cognition at Rockefeller University, is the other senior author of the study.
Both Sethupathy and Rajasethupathy are siblings and conceptualized this study over family dinners. The lead author of the study is Kuangfu Hsiao, a research associate at Rockefeller University.
The investigators started their work with around 200 genetically diverse mice to detect parts of the DNA that add to the observed changes in short-term memory among the mice. The team screened the mice on a short-term memory task and applied genetic mapping methods to identify an area of the genome—harboring a total of 26 genes—that is linked to working memory.
With additional genome-scale analyses, the researchers whittled the list of genes down to four of special interest. They later deactivated each of these four genes one at a time and identified that one specific gene, Gpr12, coded for a protein that is needed for and supports the working memory.
Rajasethupathy added, “I expected the prefrontal cortex would be the region most globally changed by the activity of Gpr12. Strikingly, it was actually the thalamus, by far.”
The team also observed that when they took low-performing mice and boosted the amount of the Gpr12 protein in the thalamus, their precision in the memory task increased from 50% to 80%, analogous to the level of high- performing mice.
To interpret the neural circuits involved, the team compared low performers against low performers with artificially increased Gpr12 protein in the thalamus. Furthermore, these mice were engineered with fluorescent calcium sensors that illuminate when a neuron is active.
The team then recorded neurons firing in various parts of the brain when the mice carried out the memory task. During several phases of the task, especially when short-term memory was needed, the researchers observed synchronous activity between the thalamus and prefrontal cortex.
When the thalamus activity went down, prefrontal went down; when the thalamus went up, prefrontal went up. We found that these two brain regions are very highly correlated with each other in high-performers but not in low-performers. This finding implies a directionality [where one area influences the other], but we don’t yet know the direction.”
Priya Rajasethupathy '04, Study Senior Author, Rockefeller University
Most often, when researchers discover that a certain gene is associated with specific behavior, it takes time and more studies to interpret how that gene is fueling the behavior.
We were inspired in this study to link genetics to neural circuits to behavior. Future work will investigate what mechanisms regulate the Gpr12 gene and what signaling pathways downstream of the Gpr12 protein mediate its effects.”
Praveen Sethupathy '03, Study Senior Author and Associate Professor of Biomedical Sciences, College of Veterinary Medicine, Cornell University
Fascinatingly, the Gpr12 gene is extremely conserved among mammals, such as humans. Therefore, the study offers the possibility of a new therapeutic angle for reversing deficits in short-term memory.
But right now, it adds a new dimension to traditional models by highlighting the significance of a two-way neural dialogue between the thalamus and the prefrontal cortex in support of short-term memory.
Source:
Journal reference:
Hsiao, K., et al. (2020) A Thalamic Orphan Receptor Drives Variability in Short-Term Memory. Cell. doi.org/10.1016/j.cell.2020.09.011.