According to a new study reported in the Oncogene journal, the thymoquinone (TQ) compound selectively destroys prostate cancer cells at advanced phases. Headed by Kanazawa University scientists, the study has reported that prostate cancer cells that lack the SUCLA2 gene can be therapeutically targeted.
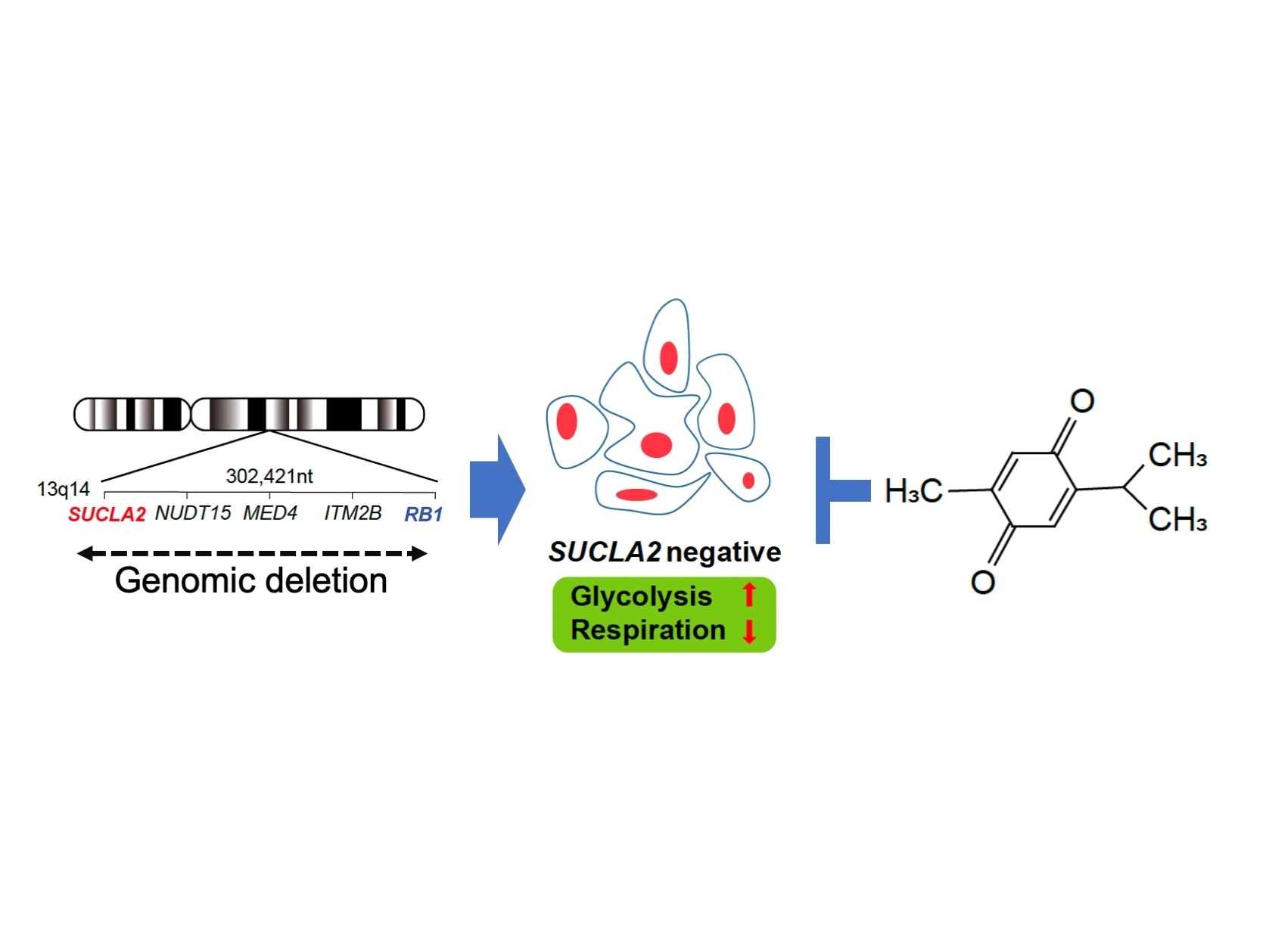
SUCLA2 gene is frequently involved in the deletion of RB1 gene region, which occurs in 10 - 30 % of advanced prostate cancers. SUCLA2 deletion gives rise to a metabolic vulnerability. By screening chemical compounds, thymoquinone appeared to selectively kill SUCLA2-deficeint prostate cancer cells. Image Credit: Kanazawa University.
In this context, SUCLA2-deficient prostate cancers denote an important fraction of those that are either metastatic or resistant to hormone therapy, and a novel therapeutic option for this disorder would benefit the patients considerably.
Although hormone therapy is usually selected for treating metastatic prostate cancer, almost 50% of patients develop resistance to this therapy within a period of just two years.
A mutation in a tumor suppressor gene—called RB1—that regulates the growth of cells has been pegged as a specifically robust driver of treatment resistance and predicts unfavorable outcomes in patients.
Mutations in tumor suppressor genes are enough to induce initiation and malignant progression of prostate cancer, but so far we haven’t been able to directly target these mutations with drugs to treat prostate cancer. We wanted to find a genetic aberration associated with that of a tumor suppressor gene which we could target therapeutically.”
Susumu Kohno, Study Lead Author, Kanazawa University
Within the genome, SUCLA2 neighbors the RB1 gene. An investigation of prostate cancer cells revealed that cells that lacked the RB1 were also missing the SUCLA2 gene, coupling the RB1 deletion with the SUCLA2 deletion present in the advanced phase of prostate cancer. Kohno and collaborators then examined prostate cancer tissue and observed that 11% of cases lacked both RB1 and SUCLA2.
The team screened compounds to detect the medications that would selectively destroy SUCLA2-deficent cells. From around 2,000 compounds, the TQ compound emerged as a target compound. This compound is known to have anti-cancer effects and was demonstrated to be safe in a phase I clinical trial.
Kohno and collaborators applied the TQ therapy to a mouse model of SUCLA2-deficient prostate cancer and found that TQ was able to selectively inhibit the growth of tumors.
These findings show that TQ treatment could be an effective therapy for treating prostate cancer cells that harbor SUCLA2 deficiency.”
Chiaki Takahashi, Study Senior Author, Kanazawa University
In a quest for genetic databases from patients suffering from prostate cancer, the team observed that the frequency of SUCLA2 loss was most perfectly aligned with RB1 loss at each phase of disease—indicating that the SUCLA2 deletion can potentially would identify patients with prostate cancer who require advanced treatment.
Identifying this drug-targetable vulnerability gives a new hope to overcome the barrier of treatment resistance for patients with prostate cancer. Additional studies need to be conducted to enhance the efficacy of the TQ compound and identify patients who would gain from this type of therapy; however, the compound also offers a promising way for new therapeutic options for advanced prostate cancer.
Source:
Journal reference:
Kohno, S., et al. (2020). Pharmacologically targetable vulnerability in prostate cancer carrying RB1-SUCLA2 deletion. Oncogene. doi.org/10.1038/s41388-020-1381-6.