It might appear to be a little game at the molecular scale. Heart muscle cells contain filament-like proteins that should have the same precise length, so that they can perfectly synchronize to facilitate the beating of the heart.
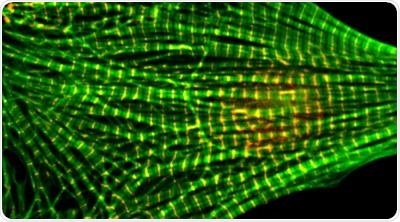
A microscope photograph of a heart muscle cell. The regular green patterns show stained actin filaments. Image Credit: Washington State University.
There is another protein that decides when the filament is the correct size and places a tiny cap on it. However, if that protein makes an error and places the cap on too early, another protein, called leiomodin, appears and displaces the cap from the way.
This kind of dance that occurs at the molecular scale may sound unimportant, but it plays a crucial role in the development of healthy muscles, including the heart. For the first time, a research team from Washington State University (WSU) has demonstrated the working of the mechanism. They have reported the study in the Plos Biology journal.
The discovery may someday result in better diagnostics and medical therapies for serious and at times debilitating hereditary heart conditions caused by genetic mutations in the proteins.
Cardiomyopathy is one of these conditions that impacts as many as one in 500 individuals worldwide and can often be deadly or have health consequences that last for a lifetime. An analogous condition, known as nemaline myopathy, impacts skeletal muscles across the body with usually debilitating consequences.
Mutations in these proteins are found in patients with myopathy. Our work is to prove that these mutations cause these problems and to propose strategies for treatment.”
Alla Kostyukova, Associate Professor and Project Leader, The Gene and Linda Voiland School of Chemical Engineering and Bioengineering
Heart muscle is composed of small thin and thick protein filaments. With the aid of electrical signals, the rope-like protein filaments both bind and unbind in a complex and accurate architecture, enabling the heart muscle to beat and contract.
The thin filaments are composed of actin, a protein that is abundantly present in the human body. Another protein, called tropomysin, encloses itself near the actin filaments. Tropomyosin along with a couple of other proteins, called leiomodin and tropomodulin, at the end of the actin filaments serve as a kind of cap and establish the length of the filaments.
Kostyukova, whose study is focused on interpreting the protein structures, added, “It's beautifully designed.” And, firmly controlled.
To maintain the health of heart muscles, all the actin filaments, which measure around a micron in length, have to be the same precise length. In families who have cardiomyopathy, gene mutations lead to the formation of filaments that are either too long or too short. The affected individuals can have major heart issues that cause illness, disability, and death.
In a study that took seven years, the team demonstrated that leiomodin binds to the end of the actin filament and knocks out the other protein, tropomodulin, to ensure the proper length of the actin filament.
This is the first time that this has been shown with the atomic-level precision. Previously, several laboratories attempted to solve this problem with very little success. With our data we finally have a direct proof.”
Dmitri Tolkatchev, Study Lead Author and Research Assistant Professor, The Gene and Linda Voiland School of Chemical Engineering and Bioengineering
The team used advanced methods to make the major proteins and analyze them at the cellular and molecular level. In this study, the molecules were designed and built at the gene level in a plasmid, and subsequently produced into cardiac or bacterial cells.
Nuclear magnetic resonance, which operates on the same physical principle as magnetic resonance imaging (MRIs), was used by the researchers to interpret the binding of proteins at the atomic level. They also employed molecular dynamic simulation to model the proteins.
The probability of being able to show this mechanism was not high, but the impact of the discovery is. This was a very important problem to study and could have a significant impact in the field of muscle mechanics.”
Dmitri Tolkatchev, Study Lead Author and Research Assistant Professor, The Gene and Linda Voiland School of Chemical Engineering and Bioengineering
Tolkatchev is also an expert in nuclear magnetic resonance.
The team hopes to pursue the work, identifying more components and molecular mechanisms that control the architecture of thin filaments, whether healthy or diseased.
Source:
Journal reference:
Tolkatchev, D., et al. (2020) Leiomodin creates a leaky cap at the pointed end of actin-thin filaments. PLOS Biology. doi.org/10.1371/journal.pbio.3000848.