Recently, researchers explained that infection by certain enteroviruses—a genus of viruses that often cause diseases of variable severity—could possibly activate diabetes, even though its direct effect in vivo and its mode of action at the molecular level were unidentified.
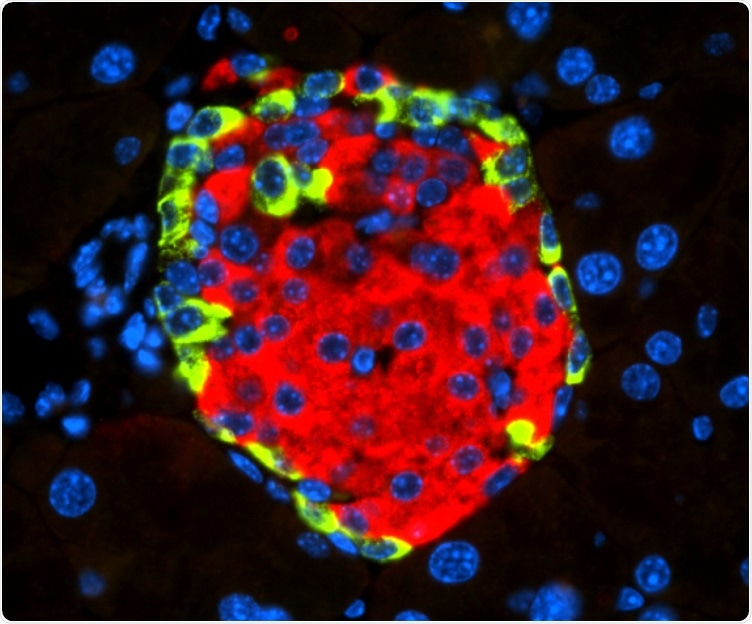
Islet of Langerhans with beta cell-secreting insulin (in red) and alpha cell-secreting glucagon (in green). Image Credit: Spanish National Cancer Research Centre.
A research team from the Growth Factors, Nutrients, and Cancer Group, headed by Nabil Djouder at the Spanish National Cancer Research Centre (CNIO), shows for the first time in Cell Reports Medicine how the enterovirus coxsackievirus type B4 (CVB4) could cause diabetes. The study results could be the stepping stone in the search for novel therapeutic treatments.
The team also indicates that the outcomes could be of significance for the COVID-19 pandemic, as clinical data suggest a possible link between diabetes and SARS-CoV-2 viral infection. Djouder and his group propose that since the SARS-CoV-2 receptor is expressed in the endocrine pancreas, it could function and cause diabetes in a manner similar to CVB4, independent of immune reactions.
Molecular mechanisms of failure in insulin production
Coxsackieviruses belong to the class of Enterovirus, which also includes echovirus and poliovirus, and which can lead to mild flu-like illnesses to more serious illnesses like meningitis, pericarditis, myocarditis, or pancreatitis. It was assumed that these viruses can lead to diabetes in humans; however, molecular mechanisms were not known.
The team from CNIO wanted to identify and explain these mechanisms and collaborated with animal models engrafted with human pancreatic cells infected by CVB4, and with mouse and human insulin-producing cells, also infected with this virus.
The researchers observed that CVB4 infection causes deregulation of URI, which is a protein that governs the normal functions of various cellular activities.
In this case, URI downregulation triggers a cascade of molecular events leading to modification of the genome via hypermethylation and silencing of Pdx1. This is a gene critical for the identity and the function of beta cells present in the endocrine pancreas, at the so-called Islets of Langerhans, and responsible for the production and secretion of insulin, a hormone that decreases blood glucose levels.”
Nabil Djouder, Study Lead Author, Spanish National Cancer Research Centre
“PDX1 silencing causes the loss of the identity and function of the beta cells, which become more like alpha cells, in charge of increasing blood glucose levels, and hence leading to hyperglycemia and subsequent diabetes, independently of any immune reactions,” Djouder.
The study was published in the Cell Reports Medicine journal.
The team demonstrated the study results by using different genetically engineered mouse models and genomic studies. The researchers reveal that loss of URI in mouse pancreata modifies the identity and function of beta cells that cause diabetes. Moreover, the team found that diabetic mice with overexpressed URI in beta cells are more tolerant to glucose.
Lastly, the team showed in several pancreata from diabetic patients that expression of URI, viral particles, and PDX1 correlates in beta cells, indicating a causal link between diabetes and enterovirus infection in humans.
The CNIO outcomes could help gain better insights into the pathological effects of the virus that causes the current pandemic.
Similarly to our investigations on enteroviruses, some recent clinical observations have associated SARS-CoV-2, the virus responsible for COVID-19, to diabetes in infected patients. Since the receptor of SARS-Co-V2 is present in beta cells, it would be interesting to study if this virus also alters URI function and silences the expression of PDX1 to affect beta-cell function, promoting diabetes.”
Nabil Djouder, Study Lead Author, Spanish National Cancer Research Centre
The team also proposes that with these findings, potential prevention, and therapeutic approach would be to use, together with anti-viral therapies, inhibitors against DNA methylase transferase, which is a protein that causes the genome hypermethylation and silencing of Pdx1.
In fact, Djouder and colleagues showed that this family of inhibitors restored PDX1 expression and glucose tolerance in diabetic mice. Many of these inhibitors have earlier been licensed for clinical use in the treatment of cancer, which could accelerate their use in such cases.
Source:
Journal reference:
Bernard, H., et al. (2020) Coxsackievirus B Type 4 Infection in ß Cells Downregulates the Chaperone Prefoldin URI to Induce a MODY4-like Diabetes via Pdx1 Silencing. Cell Reports Medicine. doi.org/10.1016/j.xcrm.2020.100125.