Each biological process depends on pH in one way or another. The human body as well as those of other organisms should maintain constant and specific pH regulation to function efficiently.
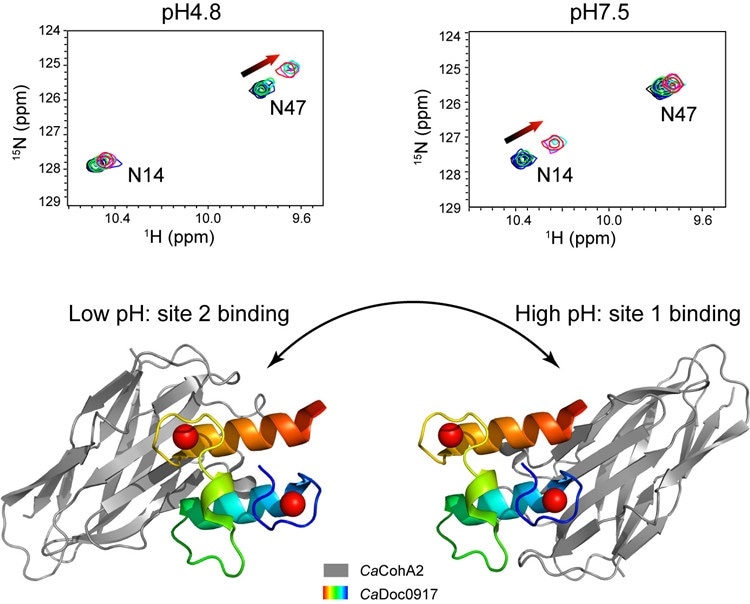
A pair of protein modules show a pH-dependent dual-binding-site switch. Upper panel: the different interaction sites observed by NMR under different pH conditions. Lower panel: the crystal structures of the complex under two pH conditions have different binding-sites. Image Credit: FENG Yingang.
pH changes can lead to adverse biological consequences, or as scientists from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), the Chinese Academy of Sciences (CAS) found considerable advantages.
The latest findings were published in the Science Advances journal on October 23rd, 2020.
Cellulosomes are essentially extracellular complexes containing numerous enzymes, which are linked to the surface of a cell. The protein molecules cohesin and dockerin found within the cellulosome cellular structure were the focus of this analysis.
Cellulosomes are complex nanomachines in nature and have great values in biofuel production and biotechnology. This study is an example of the complexity and diversity of cellulosomes.”
FENG Yingang, Study Author and Professor, Metabolomics Group, Chinese Academy of Sciences
pH changes have already been demonstrated to cause “on-off” switches inside protein functions, several of which take place naturally and are crucial for life processes. This relevant phenomenon can be used in biotechnical innovations to create switches or sensors using pH-dependent biomolecules.
The latest finding on the cellulosome assembly of the bacterium Clostridium acetobutylicum, advances this possibility by switching between a pair of functional sites, instead of simply switching “on” or “off”. This paves the way for more possibilities.
Our study not only revealed an elegant example of biological regulation but also provides a new approach for developing pH-dependent protein devices and biomaterials for biotechnological application.”
FENG Yingang, Study Author and Professor, Metabolomics Group, Chinese Academy of Sciences
The team discovered that altering the pH from 4.8 to 7.5 caused the cohesin-binding sites on the dockerin molecule to switch from one site to another. This kind of switching between a pair of functional sites has never been observed in any interaction between proteins.
Isothermal titration calorimetry (ITC) and nuclear magnetic resonance (NMR) were used to explain the distinct characteristics of this interaction. The team further observed that the attraction, or affinity, between the molecules, seems to change along with the pH. Such a property is believed to be peculiar when compared to other interactions between the cohesion and dockerin and is unique, to date, to C. acetobutylicum bacteria.
These discoveries and similar upcoming ones can probably be used for creating highly complex biological switches in synthetic biology and for further advancements in the domains of biotechnology.
Next, we will continue to elucidate the structure and regulation of cellulosomes, which could provide interesting novel discoveries and new strategies to increase the efficiency of lignocellulose-based biofuel production.”
FENG Yingang, Study Author and Professor, Metabolomics Group, Chinese Academy of Sciences
“Our ultimate goal is to promote sustainable and economical lignocellulose bioconversion and bioenergy production,” Professor FENG concluded.
Source:
Journal reference:
Yao, X., et al. (2020) Discovery and mechanism of a pH-dependent dual-binding-site switch in the interaction of a pair of protein modules. Science Advances. doi.org/10.1126/sciadv.abd7182.