In this interview, Dr. Shalin Naik speaks to AZoLifeSciences about his team's latest research that led to the discovery of a new step in the development of T and B cells that could help us to better understand leukemia.
What provoked your research into immune cells?
We are essentially walking talking T-bone steaks… wet and at 37°C – otherwise perfect conditions for rotting meat. But by and large, we are healthy human beings walking around in a germ-filled environment.
Why is that? The answer is our immune system. The question of how it works has fascinated me for the last 25 years.
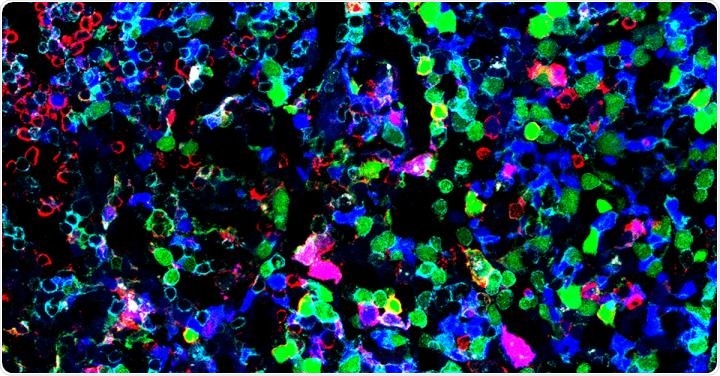
Image Credit: WEHI, Australia
What is the role of T and B lymphocytes in our immune system?
Our immune system is composed of many white blood cells, some of which are called T and B cells. These cells are part of what is known as the adaptive immune system. When the body encounters a bug it has never seen before and needs to learn its identity.
Through a lock-and-key fit system, T and B cells recognize parts of the bug which leads to them attacking and then clearing it. They also have a crucial role in killing or preventing cancer from spreading – both naturally and when harnessed in the emerging field of immunotherapy.
How are immune cells developed?
Immune cells develop from dedicated stem cells deep in our bone marrow. Here, stem cells divide in huge numbers to generate the billions of red and white blood cells required for carrying oxygen and for our immune system.
This happens through a series of highly controlled steps. Too many can lead to leukemia, too few can lead to immunodeficiency, so getting the balance right is critical.
Image Credit: CI Photos/Shutterstock.com
In your research, you identified a new step in the development of T and B lymphocytes. Can you describe how you carried out your research that led to this discovery?
Our laboratory studies the immune system using new cutting edge single-cell technologies.
Cells are the basic building blocks of all of our tissues, and understanding each cell is as crucial as understanding individual player statistics in a football match rather than just the teams’ scores.
Can you describe how multi-omics technologies work? What are the advantages of using this approach in research?
Scientists measure many different aspects of cell biology – their proteins, their DNA, the genes that are switched on and off, etc. The more features of a cell we can measure a better picture we can resolve.
In this way, we can get closer to the true nature of each cell, what it does, and how that is relevant for our biology – in our case how it functions in our immune system.
You used a single-cell multi-omics approach in your research. Do you believe that using this approach, it will encourage the use of multi-omics technologies to answer other research questions?
I really hope so, and we are just scratching the surface.
By understanding the full complement of each single cell’s function, we hope that researchers can unearth new cell types and cell states that inform health and disease.
Image Credit: Kateryna Kon/Shutterstock.com
How will your research help in future studies of the immune system?
By understanding where T and B cells come from normally as we have done in this study, we hope to use this information to think about how we might be able to boost numbers of these cell types; either in vaccination, to boost an aging immune system, or to engineer cancer-fighting immunotherapies.
Could your research help us to better understand leukemia?
Some leukemias are “lymphoid” in nature, meaning they have the same origin as progenitors that make T and B cells. Having discovered the earliest step of lymphoid development, we think this may represent the source of such cancers.
By understanding the mechanisms of our newly discovered population, we might find clues as to the origins of such leukemias.
Image Credit: Kateryna Kon/Shutterstock.com
What are the next steps in your research?
Next, we want to understand whether there are any other new previously ‘hidden’ populations that have not been discovered. This will help answer fundamental questions about the molecular mechanisms and programs by which the many different immune cell types that keep us healthy are made.
Once we have cataloged all of these, the next step will be to try out those molecular programs artificially, to ask if we can use them to synthetically produce different immune cell types for the treatment of immune and blood diseases such as cancer, autoimmunity, allergy, and vaccination.
About Dr. Shalin Naik
Dr. Naik is a graduate of the University of QLD (Microbiology & Biochemistry) where he did Honours with Prof. David Hume on macrophage activation by CpG DNA. After a 2-year hiatus in London, he returned to Melbourne to do his Ph.D. with Prof. Ken Shortman on dendritic cell development at WEHI. It was here he gained an interest in single-cell tracking and fate determination in biology and was awarded his Ph.D. in 2006.
Interested in the emerging technology of ‘cellular barcoding’ Dr. Naik did his postdoc in the laboratory of Prof. Ton Schumacher at the Netherlands Cancer Institute, where he traced the single-cell output of hematopoietic stem and progenitor cells in vivo.
After returning to WEHI in 2013, he was later appointed as a Laboratory Head in the Immunology Division where his lab uses single cell systems biology to investigate immunology, cancer, and development.
He also set up and led the Single Cell Open Research Endeavour (SCORE) at WEHI – a collaborative center for single-cell technology, experimental design, and computational projects. He has also been the co-host of Ask the Doctor on ABC and Netflix (international), and Catalyst on ABC.