The knowledge of what can be inherited from parents and how their life experiences impact children could be completely rewritten if a fundamental finding concerning a factor driving healthy development in embryos were made.
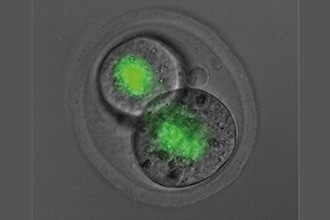 SMCHD1 produced by the mother (green) seen remaining in embryos as the cells divide. Researchers have found the effect of SMCHD1 from the mother impacts when Hox genes are activated many days later in development. Image Credit: Wanigasuriya et al., eLife 2020
SMCHD1 produced by the mother (green) seen remaining in embryos as the cells divide. Researchers have found the effect of SMCHD1 from the mother impacts when Hox genes are activated many days later in development. Image Credit: Wanigasuriya et al., eLife 2020
According to recent findings, mother-to-child transmission of epigenetic information, which is generally reset between generations and sits on top of DNA, may occur more frequently than previously believed.
The WEHI-led research widens the knowledge of which genes have epigenetic information transmitted from mother to kid and which proteins are crucial for regulating this uncommon process.
At a glance
- The mother’s egg protein that controls the epigenetic inheritance of a group of genes crucial for the development of appropriate body form in mammals was first discovered in a study.
- Although the environment, including a person’s food and exposure to contaminants, can affect their epigenome, these epigenetic alterations are extremely rarely inherited.
- The current discovery transforms the perspective of what can be passed down, suggesting that epigenetic inheritance may be more common than previously believed.
One set of genetic instructions can produce hundreds of different cell types in the body, and epigenetics studies how the genes are turned on and off to do this.
Environmental factors, such as nutrition, can affect epigenetic alterations, but DNA is not altered and epigenetic modifications are typically not transferred from parent to child.
Despite the fact that a small subset of “imprinted” genes can pass epigenetic information down the generations, very few additional genes have up until this point been demonstrated to be impacted by the mother’s epigenetic state.
The availability of a certain protein in the mother’s egg can have an impact on the genes that control the skeletal patterning of offspring, according to recent research.
The team was originally taken aback by the findings, according to chief investigator Professor Marnie Blewitt.
It took us a while to process because our discovery was unexpected. Knowing that epigenetic information from the mother can have effects with life-long consequences for body patterning is exciting, as it suggests this is happening far more than we ever thought. It could open a Pandora’s box as to what other epigenetic information is being inherited.”
Marnie Blewitt, Joint Head and Professor, Epigenetics and Development Division, Walter and Eliza Hall Institute of Medical Research
The research was led by WEHI and published in Nature Communications in collaboration with Associate Professor Edwina McGlinn of Monash University and The Australian Regenerative Medicine Institute.
Astonishing discovery
The current study concentrated on the Hox genes, which are essential for typical skeletal growth, and the protein SMCHD1, an epigenetic regulator identified by Professor Blewitt in 2008.
In mammalian embryonic development, hox genes regulate the identity of each vertebrate while the epigenetic regulator prohibits these genes from becoming active too early.
According to the findings of this study, the quantity of SMCHD1 in the mother’s egg affects the activity of the Hox genes and the patterning of the embryo. Offspring were conceived without maternal SMCHD1 and were born with altered skeletal structures.
This is unmistakable proof, according to the first author and PhD researcher Natalia Benetti, that epigenetic information rather than only blueprint genetic material was passed from the mother.
While we have more than 20,000 genes in our genome, only that rare subset of about 150 imprinted genes and very few others have been shown to carry epigenetic information from one generation to another. Knowing this is also happening to a set of essential genes that have been evolutionarily conserved from flies through to humans is fascinating.”
Natalia Benetti, Study First Author and PhD Researcher, Walter and Eliza Hall Institute of Medical Research
The study demonstrated that SMCHD1, which is only present in the egg for two days after conception, has a long-lasting effect.
Facioscapulohumeral muscular dystrophy (FSHD), a kind of muscular dystrophy, and the developmental condition Bosma arhinia microphthalmia syndrome (BAMS) are both associated with variations in SMCHD1. According to the researchers, future generations of women with SMCHD1 mutations and their offspring may be affected by their findings.
The team’s understanding of SMCHD1 is currently being used by WEHI to create novel medicines for the treatment of developmental illnesses such Prader Willi Syndrome and the degenerative disorder FSHD.
Source:
Journal reference:
Benetti, N., et al. (2022) Maternal SMCHD1 regulates Hox gene expression and patterning in the mouse embryo. Nature Communications. doi.org/10.1038/s41467-022-32057-x.