Researchers have recently discovered how the function of therapeutically relevant ion channels is regulated by drug-like small molecules. These latest findings could redefine ongoing drug development efforts.
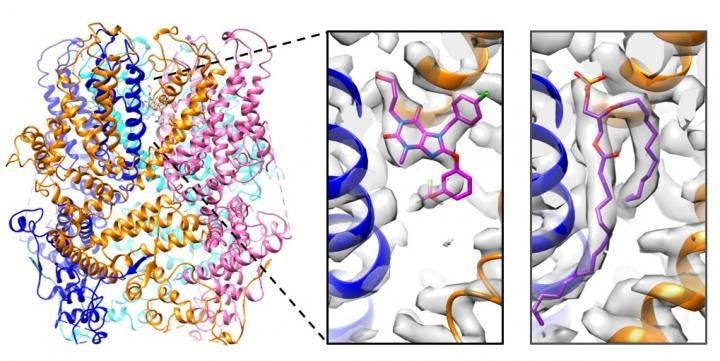
The structure of the tetrameric structure of a TRPC5 channel in complex with Pico145 (left). Each TRPC5 monomer is displayed in a different color. The structure revealed that the drug-like small molecule Pico145 can bind to each of four pockets between subunits (middle). When the structure of TRPC5 was determined in the absence of Pico145, this pocket was occupied by a phospholipid (right). Image Credit: Paper authors.
A crucial mechanism through which cells interact with their surroundings is the movement of metal ions via channels situated inside their cell membranes.
The researchers from the University of Leeds performed the new study, which was recently published in the Communications Biology journal. The study offers a comprehensive understanding of the regulation of TRPC5 ion channels, which enable positively charged ions, like potassium, sodium, and calcium, to flow in and out of cells.
TRPC5 channels are regarded as promising therapeutic targets for treating a range of medical conditions, such as anxiety, cardiovascular disease, and kidney disease.
The study—headed by Dr. Robin Bon, Associate Professor of Chemical Biology in the School of Medicine, and by Dr. Stephen Muench, Associate Professor of Membrane Biology in the School of Biomedical Sciences—shows how a drug-like small molecule, known as Pico145, attaches to the TRPC5 channel and thus prevents the channel from opening.
Using cryo-electron microscopy performed in the Astbury BioStructure Laboratory, we determined high-resolution structures of the TRPC5 channel in the presence and absence of Pico145. These structures show, for the first time, how Pico145 can displace a lipid bound to each of the four TRPC5 proteins. Further studies revealed the importance of individual amino acid residues in the Pico145 binding site of TRPC5.”
Dr David Wright, Study First Author, University of Leeds
Several diseases are associated with defects in the function of ion channels; therefore, controlling the closing and opening of certain ion channels is an extremely successful therapeutic approach.
However, drug discovery efforts are usually impeded by gaps in interpreting how drug-like small molecules can be developed to regulate the activity of ion channels.
According to Dr. Muench, “It amazes me that you think you understand how a small molecule may influence the activity of a protein - and then you find something unexpected. The displacement of a lipid opens up some interesting new directions of research into how this important family of proteins functions at a fundamental level and how we may develop new therapies in the future.”
The opening and closing of TRPC channels is regulated by many factors, including dietary components such as lipids, minerals and antioxidants, as well as environmental toxins. Overactivity of TRPC channels is linked to a range of diseases. Therefore, small molecules that can stop TRPC channels from opening are increasingly considered as potential therapeutic agents.”
Dr Robin Bon, Associate Professor of Chemical Biology, School of Medicine, University of Leeds
Dr. Bon continued, “Indeed, several pharmaceutical companies now have drug discovery programs that focus on finding new inhibitors of TRPC channels including TRPC5. Pico145, which was developed by a US-based pharmaceutical company, belongs to the most potent and selective class of molecules (called xanthines) to target TRPC5 and related TRPC channels.”
In Leeds, we have done a lot of work to understand how xanthines regulate TRPC channel activity. Our structures represent a break-through that may provide new, rational approaches to the development of drug candidates that target TRPC channels.”
Dr Robin Bon, Associate Professor of Chemical Biology, School of Medicine, University of Leeds
“In addition to its relevance to drug discovery, our study also provides new insights into how physiological and dietary factors such as lipids and zinc ions may regulate TRPC channels. Therefore, our work has opened up several new lines of research,” Dr. Bon concluded.
Source:
Journal reference:
Wright, D. J., et al. (2020) Human TRPC5 structures reveal interaction of a xanthine-based TRPC1/4/5 inhibitor with a conserved lipid binding site. Communications Biology. doi.org/10.1038/s42003-020-01437-8.