In a quest to find the genetic factors that are fundamental to diabetic retinopathy, scientists from the University of Illinois Chicago (UIC) have also discovered a new method that can be employed as a template to investigate other types of diseases.
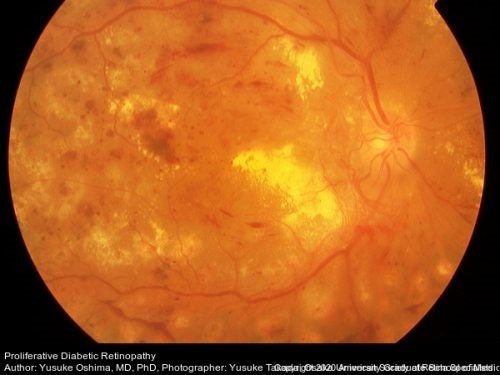
This retinal photograph shows diabetic retinopathy with neovascularization along with preretinal hemorrhage, dot and blot hemorrhages, micro-aneurysms, and cotton wool spots evident along with macular edema. Image Credit: Retina Image Bank, Dr Yusuke Oshima.
In a study titled “Integration of genomics and transcriptomics predicts diabetic retinopathy susceptibility genes” and published in the eLife journal, the investigators have detected genes that react differently in response to high glucose levels in people. Both with and without diabetic retinopathy.
Dr. Michael Grassi, associate professor of ophthalmology at UIC’s College of Medicine, his colleague Dr Barbara Stranger from Northwestern University, and their groups set out to detect genes that are responsible for causing diabetic retinopathy—a diabetes complication that occurs when light-sensitive tissue in the retina—behind the eye—are damaged. This condition can lead to vision loss.
Ever since Grassi started his clinical training as a retina specialist, he has been interested in diabetic retinopathy.
I encountered two individuals with disparate outcomes, a 19-year-old who had well-controlled diabetes for five years and went blind, and a Vietnam veteran, who had poorly controlled diabetes for over 30 years but had no vision problems.”
Dr Michael Grassi, Associate Professor of Ophthalmology, College of Medicine, University of Illinois at Chicago
For the last decade, Dr. Grassi has been studying the genetic underpinnings of diabetic retinopathy. But after many attempts, he eventually landed on a technique that allowed him to identify genes that raise the risk of developing diabetic retinopathy.
Grassi and his group integrated many different techniques to detect a gene, called folliculin, or FLCN for short; this gene raises the risk of developing retinopathy.
The researchers started by comparing gene activity levels in people. both with and without diabetic retinopathy. They also identified a set of genes that was exclusive to those with retinopathy.
The researchers subsequently took the genetic markers for this set of genes and observed that a number of these genes were linked to the development of diabetic retinopathy. They finally tested whether variations in the levels of some of these genes could account for retinopathy and found that increased quantities of FLCN increased the risk of diabetic retinopathy.
The team also investigated glucose-induced modifications in gene expression in cell lines obtained from individuals with type 1 diabetes, both with and without diabetic retinopathy. This method offers a better understanding of the disease.
To validate this finding in independent cohorts, the researchers followed the identification of single nucleotide polymorphisms (SNPs) related to such changes—that is, expression quantitative trait loci (eQtls). By using Mendelian Randomization, the team demonstrated that FLCN acts as a mediator of diabetic retinopathy, further confirming the new technique.
It has been a challenge to study diabetic retinopathy because it is so heterogeneous. There are so many genetic factors that can contribute.”
Dr Michael Grassi, Associate Professor of Ophthalmology, College of Medicine, University of Illinois at Chicago
For their analysis, the researchers used cell lines produced from blood samples obtained from the Diabetes Control and Complications Trial (DCCT)—a large clinical study of diabetic retinopathy. Since the DCCT study created cell lines for every person, it enabled a comprehensive characterization of the severity of retinopathy in each individual.
Interpreting the genetic factors involved in diabetic retinopathy can possibly result in the development of new treatment and prevention approaches for diabetic retinopathy. In the present standard of care, laser surgery is used to maintain the core part of the vision, or injections are administered into the eye every four weeks.
Source:
Journal reference:
Skol, A. D., et al. (2020) Integration of genomics and transcriptomics predicts diabetic retinopathy susceptibility genes. eLife. doi.org/10.7554/eLife.59980.