Most often, protein therapies are more selective and potent toward their biochemical targets when compared to other types of medicines, specifically small molecules. But proteins are also more likely to be rapidly degraded by enzymes or eliminated from the blood by the kidneys, and this fact has restricted their clinical application.
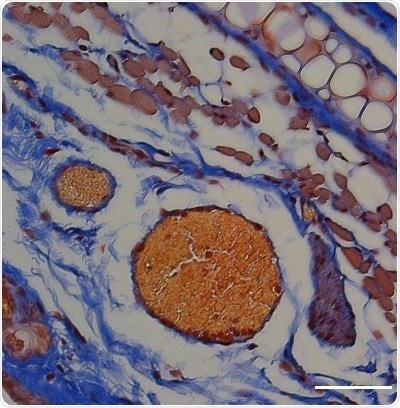
An orange-red dye shows that blood clots formed in a mouse ear in which thrombin was released from light-triggered RBCs. Scale bar, 50 μm. Image Credit: Adapted from ACS Central Science.
Now, using a honey bee peptide, scientists have designed red blood cell (RBC) carriers that discharge therapeutic proteins when activated by light. The researchers have reported their findings in the ACS Central Science journal.
Since protein drugs are volatile in the body, they should be administered at high concentrations but this, in turn, can also cause side effects. Therefore, investigators have attempted to prevent the degradation of protein therapies by encapsulating them in carriers, like nanoparticles, liposomes, and RBCs.
However, they found it difficult to make the carriers to discharge their cargo at the precise time and location. Brianna Vickerman, David Lawrence, and collaborators wanted to design RBCs to discharge therapeutic proteins at particular regions of the body when activated by specific wavelengths of light.
The team incorporated a peptide, known as melittin, into the cell membrane of RBCs. Melittin is a constituent of European honey bee venom, and it usually causes the rupture of RBCs. But the researchers altered this peptide so that it would perform the same function when lighted up by a certain wavelength of light.
As a proof of concept, the team loaded a blood-clotting enzyme, called thrombin that is used to inhibit excessive bleeding, into the engineered RBCs and then administered them in mice. Subsequently, they shined a light on a small area of each mouse’s ear and investigated the tissue sections.
The study demonstrated that thrombin-related blood clotting occurs only in the illuminated regions. This latest approach could prove handy for the light-triggered release of nucleic acid, protein, and peptide therapeutics from a wide range of lipid-based carriers, concluded the researchers.
Source:
Journal reference:
Vickerman, B. M., et al. (2020) Light-Controlled Release of Therapeutic Proteins from Red Blood Cells. ACS Central Science. doi.org/10.1021/acscentsci.0c01151.