Breast cancer is the most commonly diagnosed disease in women and is the main cause of cancer-related mortalities in this group of population. Genetic predisposition is one of the major risk factors linked to this disorder.
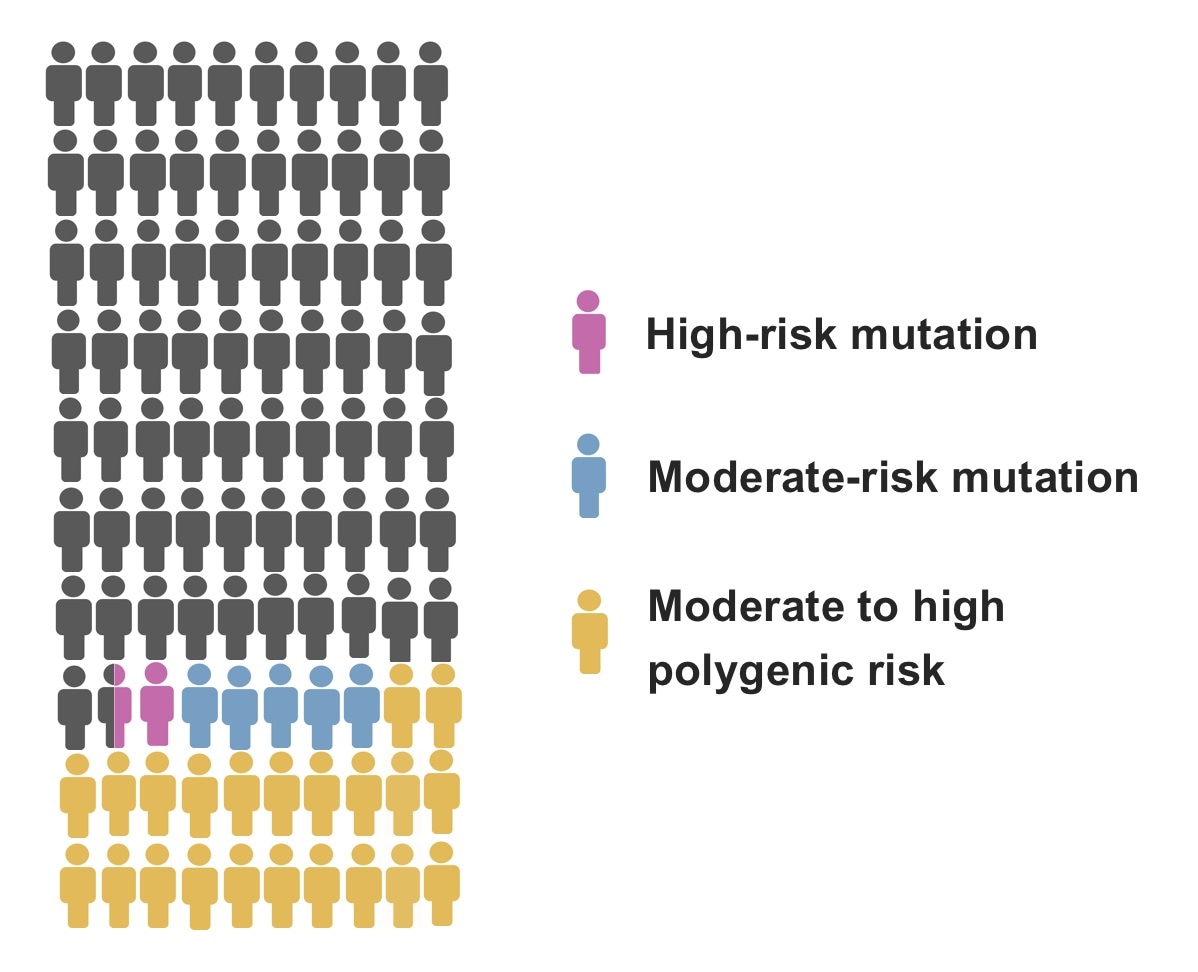
Clinical gene panel tests identify moderate-risk mutations (including mutations in the chek2 gene), and high-risk mutations (including mutations in genes PALB2, BRCA1, and BRCA2). However, moderate-to-high polygenic risk is a far more common risk factor for breast cancer, but results in a breast cancer risk at least comparable to moderate-risk mutations, And in many cases, the risk is even higher. The image illustrates the prevalence of these genetic risk factors per 100 women who have developed breast cancer. Image Credit: Nina Mars.
A research work coordinated by scientists from the University of Helsinki investigated the risk of developing breast cancer on the population level as well as in individual carriers of certain gene defects that considerably raise the risk of breast cancer.
The results, which were recently published in the Nature Communications journal, are built on the FinnGen project covering over 120,000 women; the project integrates genomic data with health data obtained from national health registries.
The research targeted particular mutations in the CHEK2 and PALB2 genes—the two most common mutations associated with high-risk breast cancer in the Finnish population. Normally, scientists use a gene test in the clinic to detect such mutations when they suspect a potent hereditary predisposition to breast cancer in the family.
The PALB2 mutation is seen in around 1% of Finnish patients with breast cancer, whereas the CHEK2 mutation is seen in almost 2.5%.
But for a long time, scientists have known that such gene defects do not lead to cancer in all women. The latest study shows that the lifetime risk of developing breast cancer among the mutation carriers differs considerably based on cumulative effects of risk factors arising elsewhere in the genome.
For the individuals, the team determined the so-called polygenic risk score, which summarizes several genetic risk factors linked with the risk of breast cancer. At the individual level, these genetic risk factors have a modest effect on the risk of developing breast cancer; however, when they are collectively integrated into a risk score, women with an unusually low or high risk of developing this disease can be differentiated from others.
For carriers of either the PALB2 or CHEK2 mutation with an elevated polygenic risk, the lifetime risk of developing breast cancer may be as high as 60–80%. Correspondingly, low polygenic risk can compensate for the impact of the mutation.”
Nina Mars, MD, Institute for Molecular Medicine Finland, University of Helsinki
The study was performed by Dr. Mars.
She continued, “For CHEK2 carriers, for example, low polygenic risk score means that their risk does not differ from that of the overall population. A more accurate risk assessment such as this for carriers of significant pathogenic mutations would help target, for example, screening for breast cancer in order to identify cases in the early stage.”
Moreover, the polygenic risk score demonstrated to be a major risk factor on its own, as the team discovered that according to the score, those in the top 10% have at least as high a risk of developing breast cancer as carriers of the CHEK2 mutation. Specifically, the polygenic risk score can offer a relatively more accurate assessment of disease risk among the close family relatives of the patient.
Even in the case of marked accumulation of breast cancer cases in individual families, clinical gene panel testing identifies a high-risk mutation in only one out of every six patients. In fact, an exceptionally high polygenic risk score, with similar impact, underlies the breast cancer risk of many families. This is why risk-related information could be used especially in assessing the risk of patients’ close relatives.”
Samuli Ripatti, Study Principal Investigator and Professor, University of Helsinki
According to the researchers, the use of polygenic risk data is a potential tool on the way toward an increasingly detailed risk evaluation for breast cancer.
Next we are starting the first trials where these scores will be integrated into the care of breast cancer patients and their families.”
Samuli Ripatti, Study Principal Investigator and Professor, University of Helsinki
“These exciting results chart a clear path towards significantly improved risk assessment that will ultimately save lives and reduce healthcare costs by ensuring that cancer screening is delivered to those who need it most,” concluded Mark Daly, the Director of the Institute for Molecular Medicine Finland.
Source:
Journal reference:
Mars, N., et al. (2020) The role of polygenic risk and susceptibility genes in breast cancer over the course of life. Nature Communications. doi.org/10.1038/s41467-020-19966-5.