Globally, breast cancer is the most common disorder in women, but despite this fact, several immunotherapies have had only minimal success in treating the aggressive forms of this disease.
New research findings from Paula Bos, PhD, identified a type of immune cells that acts as a major driver of breast cancer growth by preventing the accumulation of a specific protein that induces anti-tumor responses. Image Credit: Virginia Commonwealth University.
A deeper understanding of the immunobiology of breast cancer is critical to the success in harnessing immunotherapeutic approaches to improve breast cancer survival.”
Paula Bos, PhD, Member of Cancer Biology Research Program, Massey Cancer Center, Virginia Commonwealth University
Bos is also an assistant professor in the Department of Pathology at the Virginia Commonwealth University (VCU) School of Medicine.
Bos’ latest study results, published in the Cell Reports journal, detected a form of immune cells that serve as a key driver of the progression of breast cancer by inhibiting the buildup of a particular protein that, in turn, triggers anti-tumor reactions. This latest information could be used for developing new immunotherapeutic strategies to treat breast cancer.
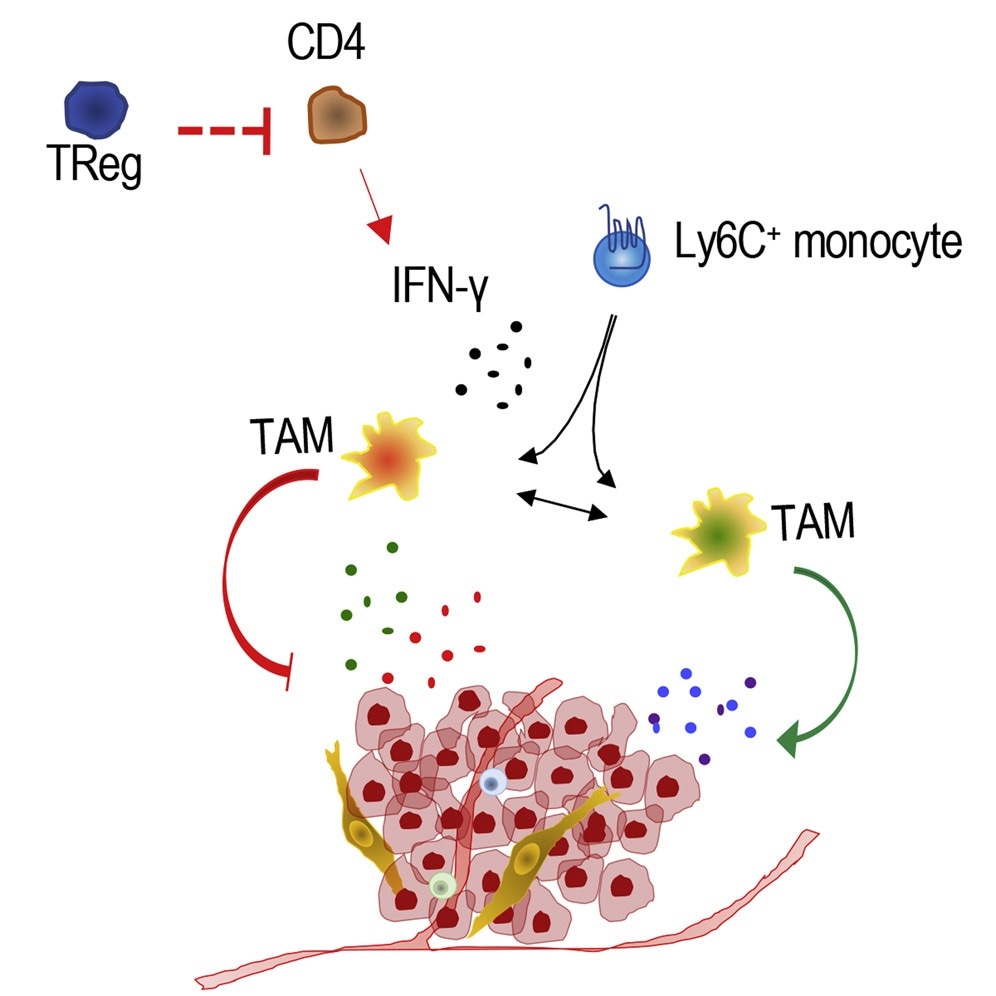
Regulatory T cells, also called Treg cells, are a unique group of immune cells that have a special capacity to inhibit the function of other immune cells. Such a function protects the organism from overreacting to particular molecules produced inside the body. But in a majority of the cases, it also suppresses the ability of the immune system to target tumor cells. Thus, Treg cells are usually prevalent in solid tumors, especially breast cancers, and are often linked with worse outcomes.
In an earlier study, Bos showed that tumor growth and metastasis can be significantly decreased by targeting Treg cells in breast cancer models; but at a molecular level, scientists are still unclear why this tumor was reducing.
Interferon-gamma (IFN-g) is a specific protein that has strong anti-tumor properties, such as the stimulation of macrophages, a type of cells that can trigger inflammation and prevent the growth of cancer.
According to the new study performed by Bos, Treg cells inhibit the production of IFN-g through CD4 T lymphocytes (a type of white blood cells), further prompting the progression of the disease.
Following the analysis of breast cancer models in which Treg cells were identified and killed, Bos observed an increased presence of IFN-g and functional reprogramming of macrophages into cancer-fighting cells.
Additionally, we demonstrated better overall survival in human cancers with similar genetic patterns to those observed in mice with breast cancer whose Treg cells were eliminated.”
Paula Bos, PhD, Member of Cancer Biology Research Program, Massey Cancer Center, Virginia Commonwealth University
This is the first-ever study to investigate the mechanistic role of Treg cells in breast cancer.
According to Bos, these results confirm the possibility for adoptive transfer therapeutics using macrophages programmed with the IFN-g protein to successfully treat breast cancer. Adoptive transfer actually involves the process of transmitting external cells into a patient to enhance the function or reaction of the immune system.
Our work raises the possibility that white blood cells can be extracted from cancer patients, reprogrammed outside of their body through brief exposure to the IFN-g protein and re-infused back into the patient, contributing to the generation of anti-tumor responses.”
Paula Bos, PhD, Member of Cancer Biology Research Program, University Massey Cancer Center, Virginia Commonwealth
Bos is presently analyzing the role of Treg cells in metastatic cancer and intends to conduct follow-up studies to test the use of IFN-g as an adoptive transfer therapeutic agent, especially in cancer mouse models.
Source:
Journal reference:
Clark, N. M., et al. (2020) Regulatory T Cells Support Breast Cancer Progression by Opposing IFN-γ-Dependent Functional Reprogramming of Myeloid Cells. Cell Reports. doi.org/10.1016/j.celrep.2020.108482.