Pictures of a protein that plays a key role in the production of a potent antibiotic have disclosed the first unusual steps involved in the synthesis of antibiotics.
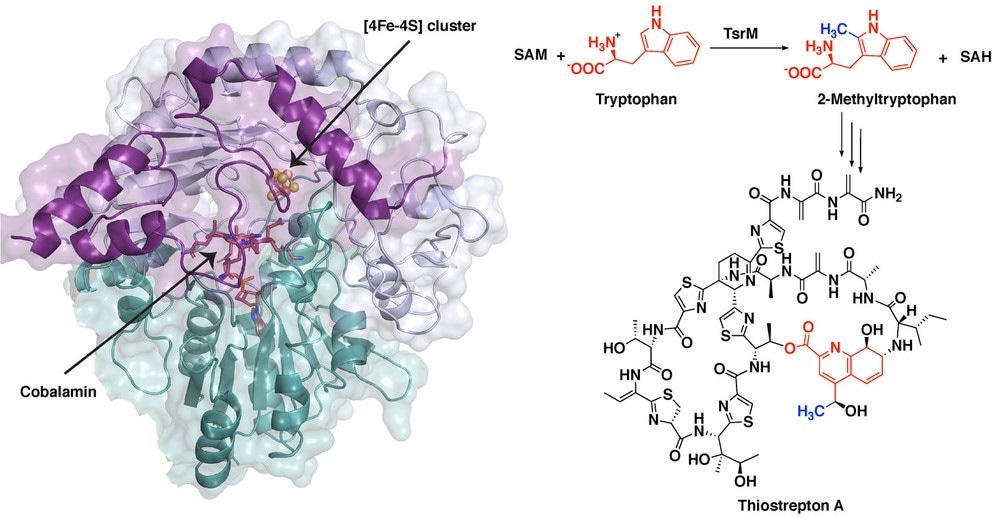
The synthesis of the potent antibiotic thiostrepton uses a radical SAM protein TsrM, whose crystal structure is shown at left while bound to an iron-sulfur cluster and cobalamin. New images of this crystal structure allowed researchers from Penn State to infer the chemical steps during the antibiotic’s synthesis (right), as a methyl group moves from a molecule called S-adenosyl-L-methionine (SAM) to the cobalamin in TsrM to the substrate tryptophan. Image Credit: Booker Laboratory, Penn State.
A better understanding of the chemistry involved in this process could enable scientists to adopt this process as well as analogous compounds for use in human medicine. The process has been described in a new study headed by Penn State chemists.
The antibiotic thiostrepton is very potent against Gram-positive pathogens and can even target certain breast cancer cells in culture. While it has been used topically in veterinary medicine, so far it has been ineffective in humans because it is poorly absorbed.”
Squire Booker, Biochemist, The Pennsylvania State University
Booker, who is also an investigator with the Howard Hughes Medical Institute, added, “We studied the first steps in thiostrepton’s biosynthesis in hopes of eventually being able to hijack certain processes and make analogs of the molecule that might have better medicinal properties. Importantly, this reaction is found in the biosynthesis of numerous other antibiotics, and so the work has the potential to be far-reaching.”
The initial step in the synthesis of thiostrepton involves a process known as methylation. Methyl group—a molecular tag—is crucial in several biological processes. This group is added to a molecule of tryptophan, that is, the reaction’s substrate.
One of the significant systems for methylating compounds that are not specifically reactive, such as tryptophan, involves a group of enzymes known as radical SAM proteins.
Radical SAM proteins usually use an iron-sulfur cluster to cleave a molecule called S-adenosyl-L-methionine (SAM), producing a “free radical” or an unpaired electron that helps move the reaction forward.”
Hayley Knox, Study First Author and Graduate Student in Chemistry, The Pennsylvania State University
Knox continued, “The one exception that we know about so far is the protein involved in the biosynthesis of thiostrepton, called TsrM. We wanted to understand why TsrM doesn’t do radical chemistry, so we used an imaging technique called X-ray crystallography to investigate its structure at several stages throughout its reaction.”
SAM directly attaches to the iron-sulfur cluster in all radical SAM proteins defined so far. Through this iron-sulfur cluster, the molecule is fragmented to create a free radical. But the team also observed that the place where SAM would usually bind is inhibited in TsrM.
This is completely different from any other radical SAM protein. Instead, the portion of SAM that binds to the cluster associates with the tryptophan substrate and plays a key role in the reaction, in what is called substrate-assisted catalysis.”
Squire Booker, Biochemist, The Pennsylvania State University
The team has presented the study results in an article published in the Nature Chemical Biology journal on January 18th, 2021.
While solving the structure, the team was able to understand the chemical steps involved during the first part of the biosynthesis of thiostrepton, when tryptophan undergoes methylation. Briefly speaking, the methyl group from SAM moves to a portion of TsrM, known as cobalamin.
This methyl group subsequently moves to tryptophan with the help of an extra SAM molecule, and thus regenerates free cobalamin and creates the methylated substrate needed for the subsequent steps involved in the synthesis of the antibiotic.
Knox added, “Cobalamin is the strongest nucleophile in nature, which means it is highly reactive. But the substrate tryptophan is weakly nucleophilic, so a big question is how cobalamin could ever be displaced. We found that an arginine residue sits under the cobalamin and destabilizes the methyl-cobalamin, allowing tryptophan to displace cobalamin and become methylated.”
The team has now planned to investigate other cobalamin-dependent radical SAM proteins to find out if they work in analogous ways. They eventually hope to create or find thiostrepton analogs that can be utilized in human medicine.
“TsrM is clearly unique in terms of known cobalamin-dependent radical SAM proteins and radical SAM proteins in general. But there are hundreds of thousands of unique sequences of radical SAM enzymes, and we still don’t know what most of them do. As we continue to study these proteins, we may be in store for many more surprises,” Booker concluded.
Source:
Journal reference:
Knox, H. L., et al. (2021) Structural basis for non-radical catalysis by TsrM, a radical SAM methylase. Nature Chemical Biology. doi.org/10.1038/s41589-020-00717-y.