A novel compound that increases lifespan in a small nematode worm has been shown to delay bone loss in aging mice. This result came from a functional and longitudinal study that involved 700 aging mice at the Buck Institute for Research on Aging.
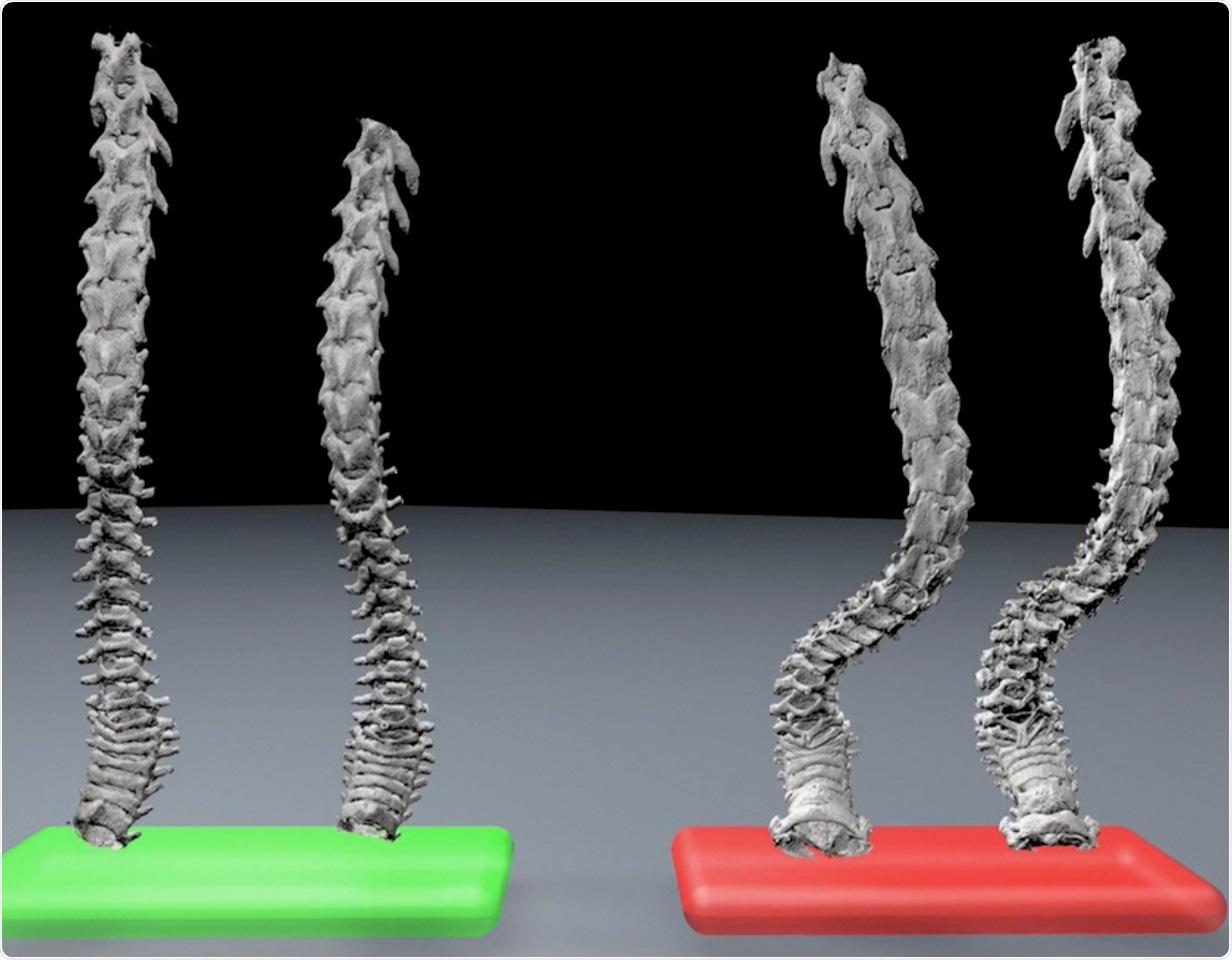
Kyphosis, Green = Young mice; Red = Old mice Image Credit: Simon Melov, PhD.
The study gives a treasure trove of information for scientists aiming to develop therapeutics to delay aging and age-related diseases. The study was recently published online in the Journal of Bone and Mineral Research Plus.
The study included five laboratories at the Buck Institute for Research on Aging and took many years to finish. In this project, the individual mice were serially profiled as they aged, and, at the same time, a number of therapeutics that prolonged the lifespan in basic model organisms, or decreased neurological disease in mice, was tested.
The team determined the rates of change for clinically relevant parameters in untreated mice. These parameters included kyphosis, activity, body composition, blood glucose, metabolic measures, and comprehensive parameters of skeletal aging in bones. In this study, terabytes of data were also collected and examined over many years.
This is a unique resource that comes from a study of multiple phenotypes of aging that had never been looked at before. Our hope is that our data will enable those working on pre-clinical studies to essentially model experiments virtually, in order to provide a starting point for testing other interventions in mice.”
Simon Melov, PhD, Study Senior Author and Professor, Buck Institute for Research on Aging
The Benzoxazole compound that delayed bone aging by as much as 31% over the course of a year’s therapy in the mice was initially identified as one of five compounds that prolonged the lifespan of the nematode in the Lithgow laboratory in a study published in the Nature journal in 2011.
If you have a therapeutic that extends lifespan in a simple animal that has no bone whatsoever, you certainly wouldn't predict that it would slow the rate of bone aging in a mammal. It's obvious that aging-related pathways have been conserved during evolution. This new finding is a great example of the utility of screening compounds in simple animals as the starting point to look for unexpected and surprising benefits in mammals.”
Gordon Lithgow, PhD, Professor and Vice President, Buck Institute for Research on Aging
In the article published in the Nature journal, Benzoxazole seemed to inhibit the aggregation of age-related proteins. This mechanism of action in a mouse bone is still being studied, even though the compound seems to delay the reabsorption of osteoclasts—bone cells that are active at the time of growth and healing—the researchers added.
According to Melov, the findings in this major study are applicable to human beings, particularly with regard to pre-clinical phenotypes. “The metrics we used are all directly applicable to aging in humans. They literally have direct clinical correlates to the types of things you would measure in humans,” Melov added.
For instance, the team has observed spontaneous fractures in the femurs of aging mouse, for the first time. According to Melov, these fractures took place in 2.5% of the mouse population, not unlike the 1%-2.7% incidences of hip fractures in individuals over the age of 65.
Melov also pointed out that the researchers have developed a new unbiased technique for assessing kyphosis—an age-related curvature of the spine—that may offer new opportunities for testing novel interventions.
We think using this new database could save substantial resources for those wanting to do pre-clinical studies of interventions. If someone wants to test a compound against a particular aging phenotype this database could provide information about how many mice are needed for the experiments and how long it would likely take to see results.”
Simon Melov, PhD, Study Senior Author and Professor, Buck Institute for Research on Aging
Source:
Journal reference:
Evans, D. S., et al. (2021) Longitudinal Functional Study of Murine Aging: A Resource for Future Study Designs. Journal of Bone and Mineral Research Plus. doi.org/10.1002/jbm4.10466.