Scientists from the Agency for Science, Technology and Research’s (A*STAR) Genome Institute of Singapore (GIS) have created a CRISPR-based gene editor named C-to-G Base Editor (CGBE) that can correct mutations responsible for genetic disorders.
The C-to-G base editor (CGBE) converts C in genes to G. This invention corrects disease-causing mutations into healthy versions, enabling treatment for genetic diseases. Image Credit: Agency for Science, Technology and Research (A*STAR), Genome Institute of Singapore (GIS).
The study was published on March 2nd, 2021, in Nature Communications.
Across the world, 1 in 17 people suffers from a certain kind of genetic disorder. There are chances for an individual or someone they know—a friend, relative, or colleague—to be one of about 450 million people affected globally. Mutations that cause these disorders can be due to multiple mutagens—ranging from spontaneous errors in cells to sunlight.
Thus far, the most common mutation is the single-based substitution, where a single-base in the DNA (for example, G) is substituted by another base (for example, C). Numerous cystic fibrosis patients across the globe have C instead of G, resulting in defective proteins causing the genetic disease. In another instance, substituting A with T in hemoglobin leads to sickle cell anemia.
To fix these replacements, the researchers created a new CRISPR-based gene editor that accurately changes the defective C in the genome to the preferred G. This C-to-G base editor (CGBE) creation paves the way for treatment options for about 40% of the single-base replacements associated with human diseases like the above-mentioned cystic fibrosis, neurological disorders, musculoskeletal diseases, and cardiovascular diseases.
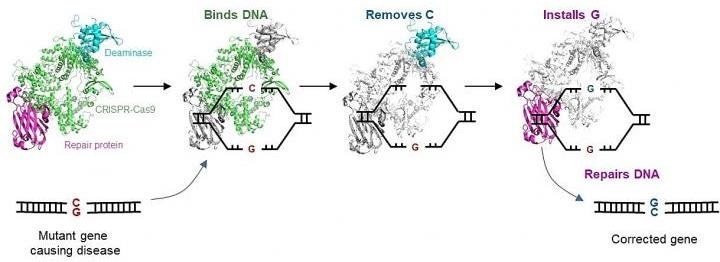
The CGBE editor optimizes the extensively used CRISPR-Cas9 technology to allow molecular surgery on the human genome. Although the CRISPR-Cas9 technology is often used to interrupt target genes, it is inefficient when an accurate change to specific sequences is preferred.
The CGBE editor offers a solution to the main aspect of this difficulty by facilitating accurate and efficient genetic modifications. CGBE includes three parts: (1) a modified CRISPR-Cas9 will indicate the mutant gene and focus the entire editor on that gene; (2) a deaminase (an enzyme that eliminates the amino group from a compound) will then target the defective C, and mark it for substitution; and (3) lastly, a protein will activate cellular mechanisms to substitute that defective C with a G.
This allows a previously unrealizable direct conversion from C to G, thus fixing the mutation and thus treating the genetic disorder.
The CGBE gene editor is a ground-breaking invention that for the first time, directly converts C to G in genes, which potentially opens up treatment avenues for a substantial fraction of genetic disorders associated with single-nucleotide mutations.”
Dr Chew Wei Leong, Senior Research Scientist, GIS
“The safety of patients is critical,” Dr. Chew reiterated. “We are working to ensure our CGBE and CRISPR-Cas modalities are both effective and safe in disease models before we can further develop such modalities for the clinic.”
Dr. Chew was one of the three young researchers to be awarded the prestigious Young Scientist Award (YSA) 2020 for his scientific efforts in gene-editing therapy.
Novel editors such as CGBE are expanding the growing suite of precise genome-editing tools that include cytidine base editors (CBEs), adenine base editors (ABEs), CGBEs, and prime editors. Together, they enable the precise and efficient engineering of DNA for research, biological interrogation, and disease correction, thereby ushering a new age of genetic medicine.”
Patrick Tan, Professor and Executive Director, GIS
Source:
Journal reference:
Chen, L., et al. (2020) Programmable C:G to G:C genome editing with CRISPR-Cas9-directed base excision repair proteins. Nature Communications. doi.org/10.1038/s41467-021-21559-9.