Imagine using only a finger to heal a wound! It seems like science fiction, reminding one of the 1982 movie E.T. Researchers have now found that the body’s own cells can perform something similarly unpredictable.
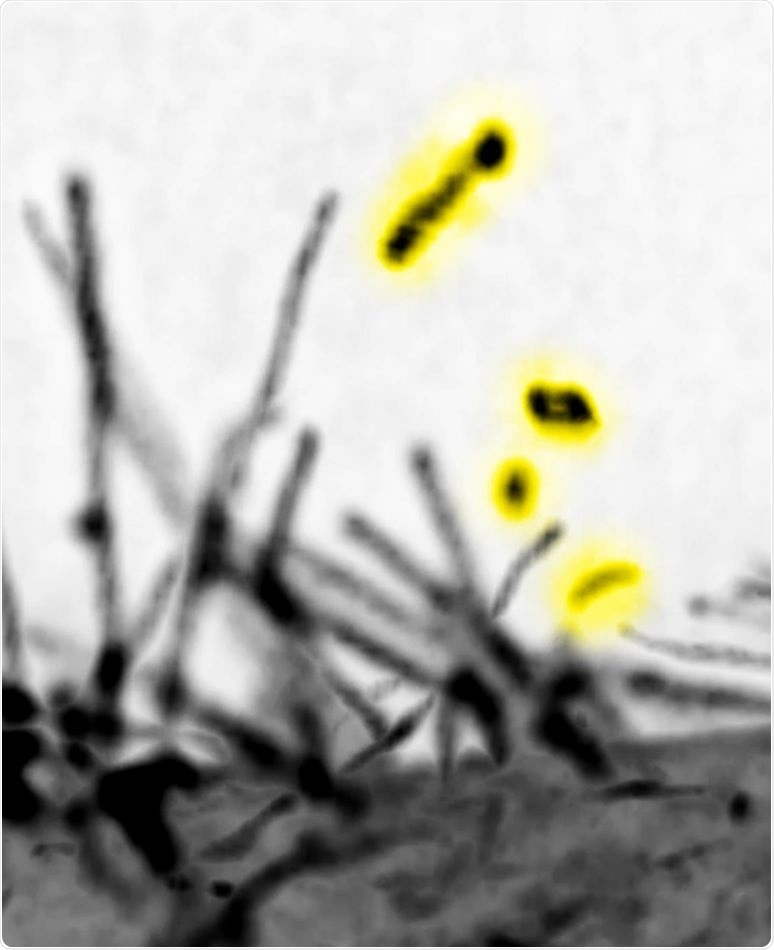
The lattice light-sheet microscopic images of the filopodia by expressing the I-BAR domain protein MIM. The vesicles that were released by the scission of MIM-induced filopodia are highlighted by yellow. The microscope locates at Mimori-Kiyosue lab (RIKEN). Image Credit: Yuko Mimori-Kiyosue and Shiro Suetsugu.
In a new study published in the journal Developmental Cell, scientists from the Nara Institute of Science and Technology (NAIST) describe a technique through which cells might use “fingers” to communicate instructions for wound closure.
Shiro Suetsugu, project leader at NAIST, has dedicated his career to the investigation of how cells shape themselves, initiate, and accept communication among each other. An under-rated way of performing this is through filopodia, small finger-like cellular projections more commonly observed to support some types of cells to crawl in the body.
Filopodia are well-recognized as cellular locomotion machinery. Less understood is how filopodia help cells communicate, and the molecular details of how this is done.”
Shiro Suetsugu, Project Leader, Nara Institute of Science and Technology
This line of study should focus on the proteins described by the acronym I-BAR. It is well known that I-BAR proteins enable bending of the plasma membrane—the “skin” of several cells—for the formation of filopodia, thereby enabling movement.
“We identified an I-BAR protein that severs filopodia,” added Suetsugu. A crucial element of this scission might be a mechanical force, which is a stimulus commonly applied by the body to cells.
Laser experiments showed that the force required for scission is approximately 8-20 kilopascals. These forces are similar to the 4-13 kilopascals, experienced by cells in blood capillaries.”
Shiro Suetsugu, Project Leader, Nara Institute of Science and Technology
Severed filopodia tend to form structures known as extracellular vesicles, a famous research topic in biology. In general, extracellular vesicles were regarded as the trash bags of cells, used for cellular waste disposal.
But now, the vesicles are regarded as communication packets instead of waste bags. “The pertinence of these vesicles to cancer metastasis has piqued researchers’ and clinicians’ interest,” explained Suetsugu.
What is the connection between this and cell-cell communication? A simulated cell-scale wound was found to heal quicker upon being treated with filopodia-derived extracellular vesicles than when left untreated.
Put differently, an I-BAR protein initially triggered filopodia scission and then the production of vesicles. The vesicles then transmitted cellular signals that boosted the migration of cells toward one another, in a manner that might support wound closure.
Suetsugu believes that by gaining insights into how cells completely use their molecular machinery to transmit instructions to other cells, medical practitioners can devise new approaches for the safe treatment of cancer and other diseases.
Certain BAR proteins are pertinent to cancer cell biology. BAR proteins are also pertinent to cell locomotion. By learning more about how these proteins aid cell-cell communication, we may find better ways to stop cancer cells from spreading.”
Shiro Suetsugu, Project Leader, Nara Institute of Science and Technology
Source:
Journal reference:
Nishimura, T., et al. (2020) Filopodium-derived vesicles produced by MIM enhance the migration of recipient cells. Developmental Cell. doi.org/10.1016/j.devcel.2021.02.029.