Researchers from the Institute for Integrated Cell-Material Sciences (iCeMS) and collaborators in Japan have uncovered molecular mechanisms involved in removing unwanted cells in the body.
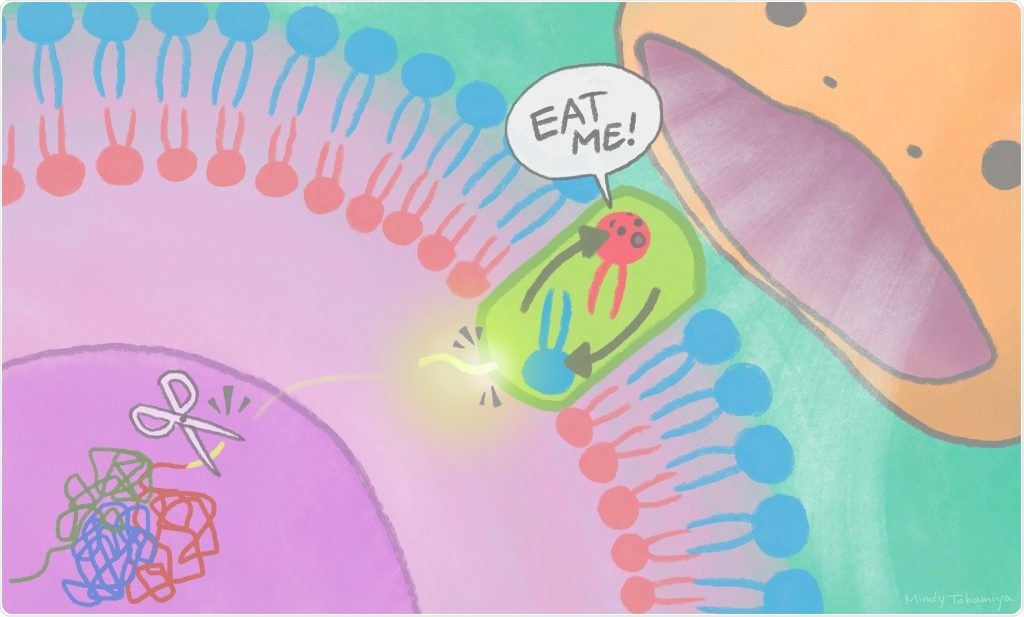
Cut-away pieces of XRCC4 protein travel out of the nucleus to the cell membrane to activate scramblases, turning on an “eat me” signal recognized by phagocytes. Image Credit: Mindy Takamiya/Kyoto University iCeMS.
A fragment of nuclear protein discharged into the cytoplasm stimulates a plasma membrane protein to exhibit a lipid on the surface of a cell, signaling other cells to eliminate it. The study results were published in the Molecular Cell journal.
Every day, ten billion cells die and are engulfed by blood cells called phagocytes. If this didn’t happen, dead cells would burst, triggering an auto-immune reaction. It is important to understand how dead cells are eliminated as part of our body’s maintenance.”
Jun Suzuki, Study Lead and Biochemist, Institute for Integrated Cell-Material Sciences
Researchers were already aware that dead cells exhibit an “eat me” signal on their surface that is detected by phagocytes. At the time of this process, lipids are switched between the outer and inner portions of the cell membrane through a wide range of proteins, known as scramblases.
Suzuki and his research team have already detected many of these lipid-scrambling proteins; however, a few of their activation mechanisms had continued to be vague.
To resolve this problem, the researchers employed a range of screening methods to investigate the scrambling protein, known as Xkr4. The ultimate objective was to isolate the genes that remain active at the time of cell death and to particularly zoom in on the Xkr4 protein and its related proteins to interpret their interaction.
“We found that a nuclear protein fragment activates Xkr4 to display the ‘eat me’ signal to phagocytes.”
Masahiro Maruoka, Study First Author and Cell Biologist, Institute for Integrated Cell-Material Sciences
The team has specifically discovered that cell death signals result in a nuclear protein, known as XRCC4, getting spliced by an enzyme. An XRCC4 fragment exits the nucleus, stimulating the Xkr4 protein, which forms a dimer: that is, the joining of identical pieces into configurations.
XRCC4 binding as well as dimer formation is required for the Xkr4 protein to eventually transfer lipids on the surface of the cell to warn phagocytes.
Xkr4 is just one of the scrambling proteins and others are stimulated relatively faster at the time of cell death. Currently, the researchers want to interpret why and when the Xkr4 pathway is particularly activated. Since the Xkr4 protein is robustly expressed in the brain, it is probably significant for brain function.
We are now studying the elimination of unwanted cells or compartments in the brain to understand this process further.”
Masahiro Maruoka, Study First Author and Cell Biologist, Institute for Integrated Cell-Material Sciences
Source:
Journal reference:
Maruoka, M., et al. (2021) Caspase cleavage releases a nuclear protein fragment that stimulates phospholipid scrambling at the plasma membrane. Molecular Cell. doi.org/10.1016/j.molcel.2021.02.025.