Researchers from Imperial Brands have compared vape e-liquid samples and their aerosols to combustible cigarette smoke using Toxys’ ToxTracker range of stem-cell-based in-vitro assays. These assays offer mechanistic insights into the possible DNA damaging characteristics of chemicals.
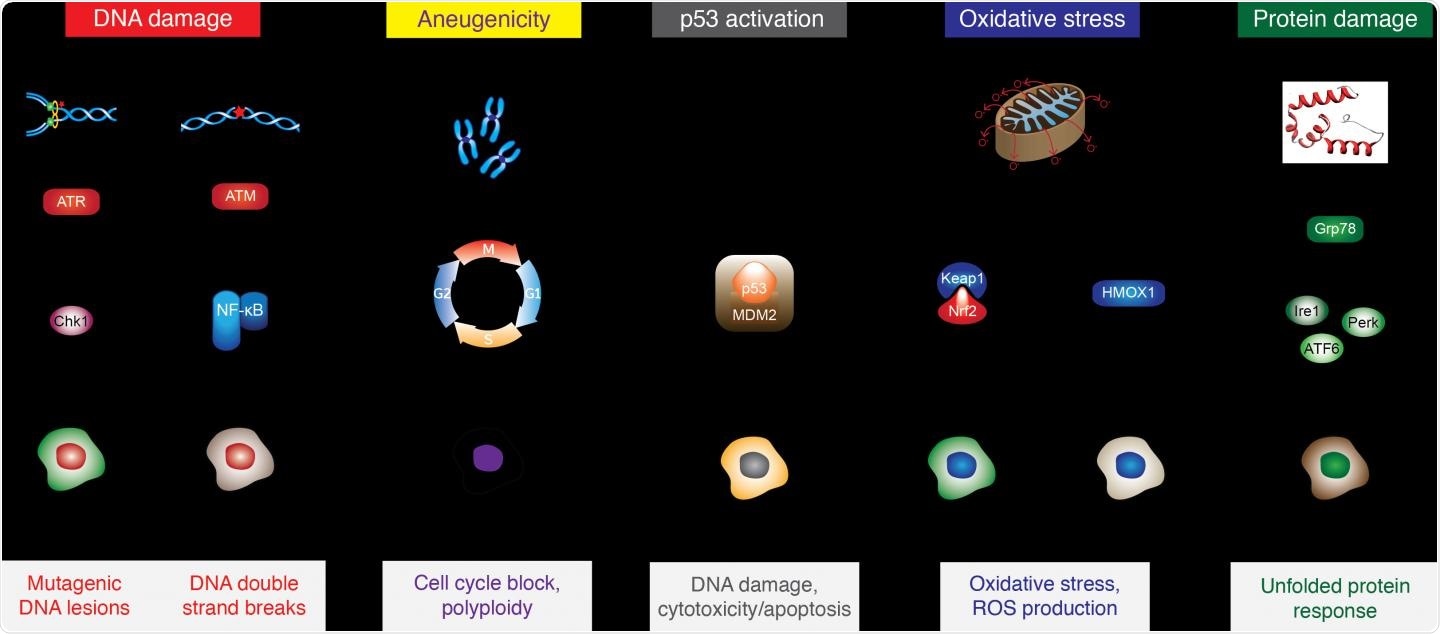
Diagram shows the six reporter cell lines of the ToxTracker test, plus a “wild type (wt)” cell line used to visualize microscopic signs of DNA damage. Image Credit: Imperial Brands.
The Imperial Brands team is the first to report the findings related to the assessment of vape e-liquids and aerosols using the ToxTracker system. This system forms a part of the company’s ongoing study on the tobacco harm reduction potential of Next Generation Products (NGPs), including vapes.
The in-vitro assays help determine how product specimens can affect cellular functioning across six reporter cell lines, detecting the tell-tale molecular signatures of possible harm in the form of protein and DNA damage, oxidative stress, and also the activation of the p53 gene that is involved in tumor suppression and cell cycle regulation.
The findings, which were peer-reviewed and published in the Mutagenesis journal, demonstrated that under the test conditions, undiluted vape e-liquids and their aerosol extracts showed completely absent or significantly reduced signs of DNA-damaging potential in cells when compared to smoke emitted by combustible cigarettes.
Overall, the data from our latest study adds to the weight of scientific evidence demonstrating vape products offer significant harm reduction potential compared to combustible cigarettes.”
Lukasz Czekala, Study Lead Author and Senior Pre-Clinical Toxicologist, Imperial Brands
Suite results
Following the calibration and validation of the system to ensure that the main e-liquid components, such as vegetable glycerine (VG) and propylene glycol (PG), were compatible with the ToxTracker range, a number of neat myblu vape e-liquids and their aerosol extracts were evaluated against smoke samples from the reference 1R6F combustible cigarette. Findings demonstrated that:
- Vape aerosols confined in a buffer solution did not trigger reactions in any of the six cell lines.
- While flavored, undiluted e-liquids (tested up to 1%) induced both oxidative stress reporters, this was believed to be an impact of osmolarity (that is, a measure of solution concentration) induced by PG/VG in in-vitro testing surrounding.
- Nicotine content did not impact responses: the same results were produced by tobacco flavor e-liquid at either 1.6% nicotine salt, 1.6% freebase nicotine, or nicotine-free.
- Nicotine (tested alone) produced only an oxidative stress response at levels that would be over 40,000 times higher than found in the blood from regular smoking.
ToxTracker is quick, sensitive and can provide a greater mechanistic resolution than existing Next Generation Product (NGP) stewardship DNA damage tests like the micronucleus and Ames assays.”
Dr Fiona Chapman, Study Corresponding Author and Pre-Clinical Toxicologist, Imperial Brands
“Our adoption of this cutting-edge in-vitro suite reinforces our commitment to using advanced cellular assays which adhere to Toxicity Testing in the 21st Century (TT21C) principles. This also contributes to reducing industry reliance on in-vivo (animal) experiments,” Dr. Chapman added.
According to Dr. Grant O’Connell, the Head of Tobacco Harm Reduction Science at Imperial Brands, “This paper adds to the established body of scientific data that shows vaping products, when manufactured to high quality and safety standards, have significant tobacco harm reduction potential relative to continued cigarette smoking.”
We appreciate society’s ongoing concerns about the health risks of smoking, and are committed to undertake high-quality research on potentially less harmful nicotine product alternatives to combustible tobacco for adult smokers.”
Dr Grant O’Connell, Head of Tobacco Harm Reduction Science at Imperial Brands
Source:
Journal reference:
Czekala, L., et al. (2021) The in vitro ToxTracker and Aneugen Clastogen Evaluation extension assay as a tool in the assessment of relative genotoxic potential of e-liquids and their aerosols. Mutagenesis. doi.org/10.1093/mutage/geaa033.