Autophagy is essential in messenger RNA (mRNA) degradation, according to researchers from the Tokyo Institute of Technology (Tokyo Tech).
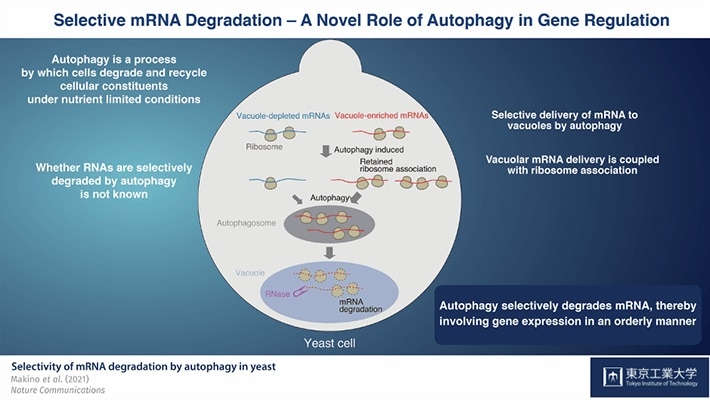
Image Credit: Tokyo Institute of Technology.
They showed, using yeast as a model system, that autophagy-mediated mRNA degradation is not a spontaneous event but a selective one in which certain mRNAs are targeted for degradation. Surprisingly, this mechanism is closely linked to ribosomal association. As a result, the researchers discovered a novel role of autophagy in the regulation of genes.
Optimal cell function necessitates a delicate balance between biomolecule synthesis and degradation. Autophagy is the mechanism through which cells degrade and recycle their own parts, thus assisting in the cleaning and maintenance of the cell’s internal environment and ensuring the proper functioning of cellular processes.
When cells are exposed to stresses such as nutrient scarcity, autophagy is highly triggered, serving to provide nutrients via the breakdown of unnecessary cellular content. Autophagy substrates are transmitted to vacuoles in lysosomes or yeast in mammals for degradation by “autophagosomes,” which are double-membrane vesicles.
Although autophagy was initially thought to be a non-selective mechanism that isolates substrates in the cell’s cytoplasm at random, research works have shown that some cellular elements, including a subset of proteins and superfluous or damaged cell organelles, are selectively isolated.
Unlike the well-established targeting of proteins and organelles by autophagy, the issue of whether RNAs are exposed to autophagy and whether they are selectively degraded has not been solved yet.
A research team from Tokyo Tech and RIKEN performed a systematic analysis of the preferential degradation by autophagy of messenger RNAs (mRNAs), which include the information needed to render cellular protein and bind ribosomes for protein synthesis, in their latest article, which was published in Nature Communications.
Prof. Yoshinori Ohsumi of Tokyo Tech received the 2016 Nobel Prize in Physiology for Medicine for his groundbreaking research in the area of autophagy.
We have previously shown that RNA delivered to the vacuole via autophagy in yeast cells, where it is degraded by vacuolar nucleases. The question of whether RNA degradation by autophagy occurs preferentially, however, remains unaddressed. This difficult to address question was the starting point of this project.”
Yoshinori Ohsumi, Study Corresponding Author and Professor, Tokyo Institute of Technology
Since RNAs accumulating in the vacuole are degraded enzymatically by the Rny1 nuclease, the researchers first created a yeast strain without this enzyme. They were able to isolate and classify RNAs collected in the vacuole using this strain.
The researchers then used the autophagy-inducing drug rapamycin to examine the special features of mRNA species delivered to the vacuole in Rny1-deficient cells when autophagy is triggered. They found that autophagy-mediated mRNA transmission to vacuoles is selective rather than spontaneous in nature.
The researchers then identified “vacuole-enriched” and “vacuole-depleted” mRNAs by characterizing various mRNA species through a large study of the types of mRNAs found in these cells.
Housekeeping mRNAs, like those that encode proteins involved in amino acid biosynthesis, were found to be the most likely ones to be transmitted to vacuoles. On the other hand, mRNAs needed for the production of proteins with regulatory mechanisms, like protein kinases, were primarily identified in the vacuole-depleted mRNA fraction.
The researchers also showed that mRNAs in the process of translation are delivered to the vacuole, implying that this is a translation-dependent process. Furthermore, after rapamycin therapy, persistent ribosome-mRNA interaction was identified to be a primary determinant of vacuolar mRNA delivery throughout autophagy-mediated degradation.
Emphasizing the significance of autophagy in gene regulation, Dr. Makino and Prof. Ohsumi stated, “Our findings suggest that autophagy regulates mRNA degradation at the translation step, thereby enabling a rapid and sensitive switch from ribosome-associated mRNAs to expression of mRNAs that are essential for an effective response to stress. Preferential degradation of ribosome-mRNAs by autophagy is therefore very likely to determine the fate of individual mRNAs as cells adapt to new conditions.”
Source:
Journal reference:
Makino, S., et al. (2021) Selectivity of mRNA degradation by autophagy in yeast. Nature Communications. doi.org/10.1038/s41467-021-22574-6.