Researchers from UT Southwestern used a new kind of gene therapy to successfully treat mice with Duchenne muscular dystrophy (DMD), distinctively employing CRISPR-Cas9-based tools to restore a large section of the dystrophin protein missing in several DMD patients.
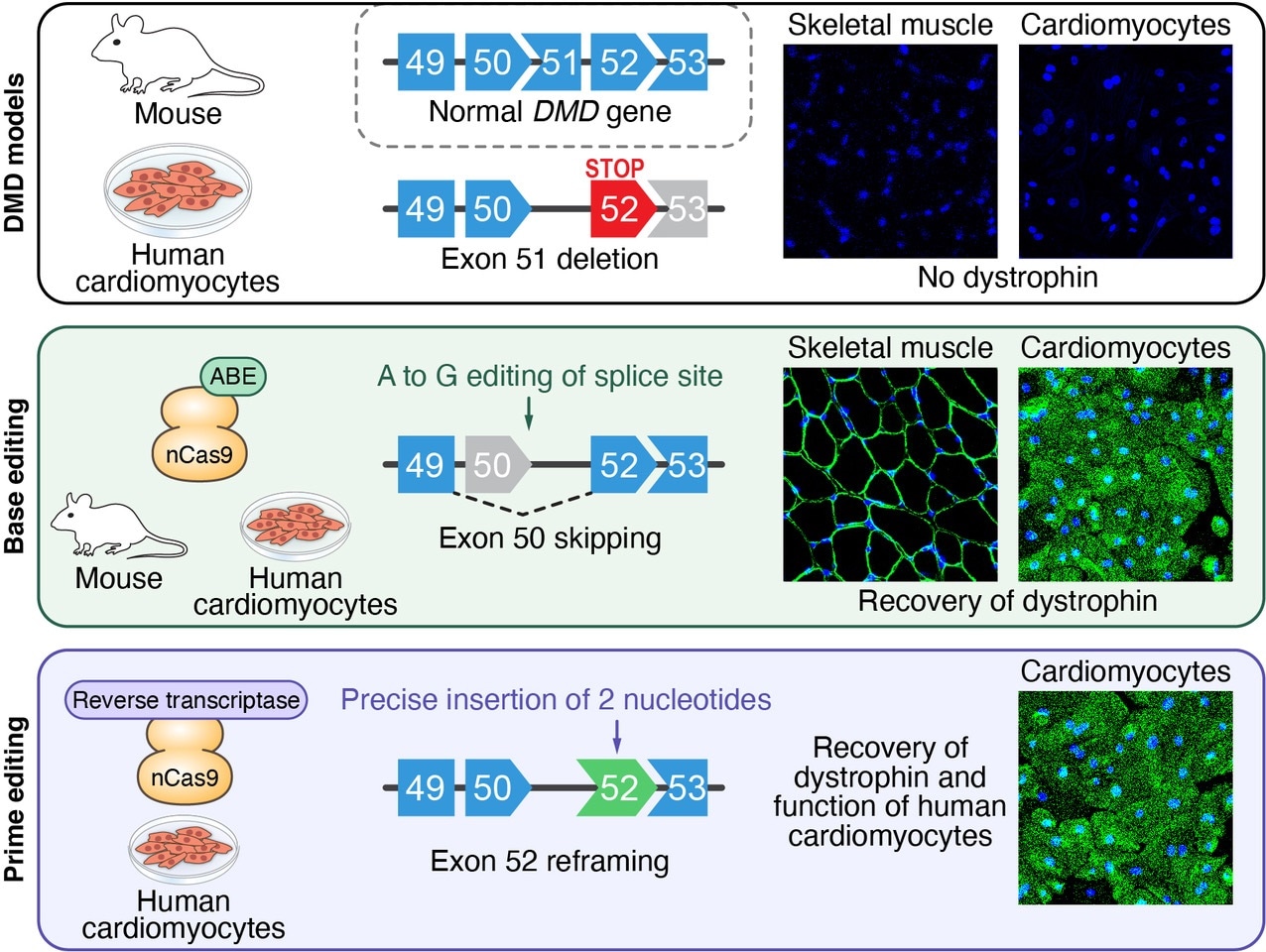
In a mouse model and in human heart muscle cells, researchers used gene editing to modify specific DNA sequences and restore dystrophin production in mutant dystrophin genes.
The new technique was reported recently online in the Science Advances journal and could enable treatment for DMD and inform the treatment of other hereditary diseases.
“Thousands of different mutations causing Duchenne have been identified, but they tend to cluster into certain parts of the dystrophin gene,” stated Eric Olson, PhD, study leader, professor, and founding chair of molecular biology at UTSW.
Certain mutations of this type make muscle cells generate short, less functional versions of the dystrophin protein.
The power of our method is that you don’t need a new gene editing strategy for every patient with a new mutation; you can correct multiple different mutations with a consolidated approach.
Eric Olson, Professor and Founding Chair of Molecular Biology, UT Southwestern
Olson and his team leveraged the fact that the very huge dystrophin gene is made of several different segments, known as exons, a few of which are dispensable. In nearly 8% of boys suffering from DMD, almost half of the dystrophin protein is missing because of mutations inside exon 51 that make the body block protein synthesis.
The team created several successful CRISPR-Cas9 nucleotide gene editing approaches to evade the errant “stop” signal, thereby restoring the synthesis of 97% of the protein. Certain approaches worked by eliminating neighboring exons, whereas others used small genetic subtractions or additions to restore the protein synthesis.
The use of the new technique in mice with dystrophin mutations led to the returning of functioning copies of dystrophin to over half of all leg muscle fibers within three weeks. The researchers also exhibited that isolated cells from mice or humans with DMD could be used to test whether the technique would apply for a specific patient ahead of treatment.
The researchers coaxed the isolated cells to form into induced pluripotent stem cells (iPS cells) and then heart cells. In a dish, they can see whether the gene-editing program enables the heart cells to work better.
Using iPS cell-derived cardiomyocytes from DMD patients, we rapidly tested our nucleotide gene editing approaches, demonstrating the recovery of the dystrophin protein.
Francesco Chemello, PhD, Study First Author and Postdoctoral Researcher in Olson Lab, UT Southwestern
DMD affects nearly 1 in 5,000 males at birth and causes progressively worsening muscle weakness in early childhood. The disease is the result of one of over 7,000 various mutations in the gene for dystrophin—a protein that usually serves as a scaffold to support muscle fibers. The lack of fully functional dystrophin causes the skeletal and heart muscles of people with DMD to degenerate over time, ultimately causing death.
The gene therapy described in this study is not yet ready for humans with DMD. More safety studies in animals are essential first, together with more studies to improve the virus carrying the gene-editing machinery to muscle and heart cells. However, by demonstrating that several CRISPR-Cas9 approaches can correct a mutation, the team has widened the toolbox of possible gene therapy options for DMD.
Every cell in the human body has 3 billion letters of DNA sequence in its genome, and this method makes it possible to correct large deletions in the DMD gene by specifically swapping one of these letters. That level of specificity and efficiency is remarkable.
Eric Olson, Professor and Founding Chair of Molecular Biology, UT Southwestern