Alzheimer’s disease is the most prevalent form of dementia distinguished by the accumulation of amyloid plaques in the brain. Microglia, the brain’s immune sentinels, are responsible not only for removing external pathogens but also for preserving brain homeostasis by clearing toxic waste like amyloid plaques.
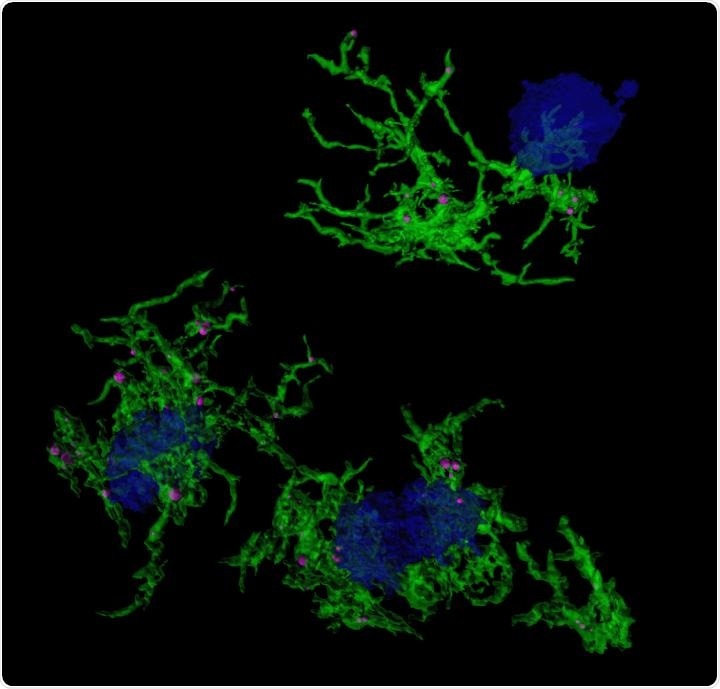
Confocal microscopic image of microglia (green) engulfing amyloid plaques (blue). Image Credit: Duke-NUS Medical School.
The function of microglia in Alzheimer’s disease and their association with amyloid plaque accumulation, on the other hand, remain unknown.
A team of researchers from Duke-NUS Medical School and Monash University has discovered the gene expression signatures underlying microglia correlated with amyloid plaque phagocytosis—the engulfment of amyloid-beta (A) protein deposits in the brain.
The results, published in Nature Communications, provide a new target for interventions aimed at addressing the underlying disease mechanism of this incurable disease.
The team of scientists at Duke-NUS and Monash embarked on an ambitious project to comprehensively research gene expression variations in various human brain cell forms that are correlated with Alzheimer’s disease development to explore the distinctions between healthy brains and those of patients with Alzheimer’s disease at the single-cell resolution.
The team focused on microglia because of the research published in Nature Neuroscience in 2019.
We sought to understand the molecular mechanisms and differences between microglia that were actively engulfing amyloid plaques in Alzheimer’s disease and those that weren’t.”
Enrico Petretto, Study Co-Senior Author and Associate Professor, Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School
The researchers achieved this by using methoxy-XO4, a stain that particularly targets microglia that have engulfed amyloid plaques. They used the stain in preclinical Alzheimer’s disease models and then looked at gene expression in the stained microglia.
They looked into the variations in gene expression that underpin microglia’s ability to digest particles (such as amyloid plaque) and discovered associated regulatory molecules.
Understanding this mechanism is important because now we have several new targets to go after, and in the future, these targets may open a new front against this devastating disease.”
Jose M. Polo, Study Co-Senior Author and Professor, Biomedicine Discovery Institute, Monash University
According to the findings, gene expression variations of microglia that have not taken up amyloid are more similar to those in aged microglia, which are considered to be dysfunctional and a significant player in Alzheimer’s disease pathogenesis.
Furthermore, after engulfing the amyloid plaques associated with Alzheimer’s Disease, microglia evolve a distinct gene expression signature or pattern. This alteration in gene expression is caused in part by the Hif1a gene.
The altered gene expression raises microglia’s capacity to pick up proteins like amyloid, while lowering Hif1a does the same, demonstrating the role of Hif1a in regulating this microglia activity. This regulatory function of Hif1a can also extend to the action of microglia in removing damaged synapses.
“It is possible that this process is initially protective, with the microglia effectively pruning damaged synapses located near plaques,” said Petretto.
However, the scientists suspect that as the disease spreads, this pruning phase will go awry. The researchers have used statistical models to simulate the networks of molecules involved in protein uptake by microglia and found new drug development targets.
Rapamycin, a commonly used immunosuppressant, was discovered to prevent the gene Hif1a from activating microglia to engulf amyloid plaques.
This relationship between Hif1a and cognitive decline in Alzheimer’s disease is yet to be comprehensively uncovered. Future work could focus on using gene editing tool CRISPR to test the impact of manipulating Hif1a on symptom severity and disease progression.”
Gabriel Chew, Study Co-First Author and PhD Student, Duke-NUS Medical School
Source:
Journal reference:
Grubman, A., et al. (2021) Transcriptional signature in microglia associated with Aβ plaque phagocytosis. Nature Communications. doi.org/10.1038/s41467-021-23111-1.