A new study performed by International School for Advanced Studies (SISSA) integrates molecular dynamics simulations and experimental data to inspect biomolecules in their natural environment.
Extended and compact structures of an RNA molecule in different solutions. Image Credit: M. Bernetti and G. Bussi.
The structure of a biomolecule can provide immense data about its interaction and function with the surrounding environment. One such evident example is the double-helical structure of DNA and its implications for the processes of transmitting genetic data.
Now, a recent study by SISSA, reveals the combination of experimental data and computer simulations of molecular dynamics to investigate the conformation of an RNA fragment implicated in protein synthesis and its reliance on the salts present in the solution.
The study has been published in the Nucleic Acids Research journal. It has resulted in the development of a new technique for a high-resolution definition of the structures of biomolecules in their physiological environments.
X-ray crystallography, as used to discover the double-helical conformation of DNA, remains one of the most common techniques for studying biomolecule structures. This technique allows us to reconstruct the image of the molecule in solid-state crystalline form. However, this yields a static view of the structure that may not correspond to that assumed in the aqueous natural environment in which biomolecules are normally found.”
Giovanni Bussi, Physicist, SISSA
This is the reason why scientists had started using the small-angle X-ray scattering (SAXS) technique in the last 10 years to analyze RNA molecules, which can have highly dynamic structures.
The new technique was directly used in aqueous solutions that mimic the physiological environment. The composition of the solutions can also be altered to investigate the adaptability of these molecules to different conditions. However, the resolution of SAXS is quite limited, in the order of a nanometer.
To overcome the limitation, Giovanni Bussi and Mattia Bernetti, a research fellow at SISSA, enhanced SAXS through a “computational microscope.” They combined it with molecular dynamics simulations that enable atomic-level computerized reconstruction of molecular structures.
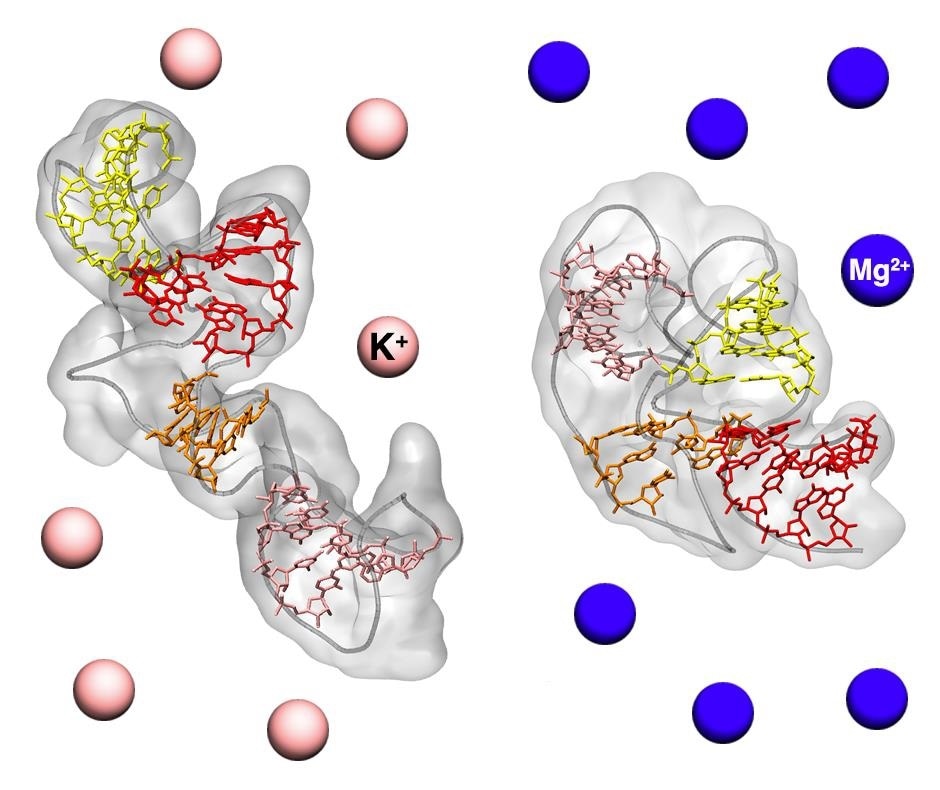
We studied a fragment of ribosomal RNA involved in protein synthesis. We used SAXS data, derived from aqueous solutions containing different mixtures of salts, that was provided by Kathleen B. Hall of the Washington University School of Medicine in St Louis, and combined them with molecular dynamics simulations. By this means we discovered the existence of two distinct conformations: one more compact and functional to the protein synthesis process, the other more extended, confirming the dynamic nature of RNA.”
Giovanni Bussi, Physicist, SISSA and Mattia Bernetti, Research Fellow, SISSA
“In particular, we noticed how the prevalence of one structure over the other varies with the salts dissolved in solution, further underlining the importance of studying these molecules in an environment as similar as possible to that of the cell,” added the team.
The researchers concluded that the study results published in the Nucleic Acids Research journal hold significance beyond this specific case and represent a novel method that provides two benefits.
In this work, we combined molecular dynamics simulations and SAXS experimental data to obtain high-resolution structures of RNA biomolecules. This is a useful approach in two senses: on one hand, it allows detail to be added to SAXS experimental data, which in fact give a very approximate view; on the other hand, it allows results of molecular dynamics to be corrected if the models used in the simulations are insufficiently accurate.”
Giovanni Bussi, Physicist, SISSA and Mattia Bernetti, Research Fellow, SISSA
Source:
Journal reference:
Bernetti, M., et al. (2021) Reweighting of molecular simulations with explicit-solvent SAXS restraints elucidates ion-dependent RNA ensembles. Nucleic Acids Research. doi.org/10.1093/nar/gkab459.